Tuning photoluminescence of single-layer MoS
2
using H
2
O
2
Weitao Su,
*
ab
Honglei Dou,
a
Jinwei Li,
a
Dexuan Huo,
*
a
Ning Dai
b
and Li Yang
c
Enhancing photoluminescence (PL) of single-layer (1L) MoS
2
is critical to its application as the thinnest light-
emitting material. In this report, we show that the PL intensity of 1L-MoS
2
can be enhanced by 8 times using
physisorption of H
2
O
2
molecules as p-type dopants. By using toluene to form the sandwiched structure of
H
2
O
2
/1L-MoS
2
/toluene, the PL intensity of 1L-MoS
2
can be enhanced up to 27.4 times. Our research
proposes a simple but effective method to enhance the light emitting properties of 1L-MoS
2
.
1. Introduction
In recent years, as the thinnest optoelectronic material, single-
layer MoS
2
has drawn much attention in various potential
applications, such as light emission,
1,2
photon-detection,
3–5
energy harvesting,
2
etc. Bulk MoS
2
crystal is composed of peri-
odic layers bonded together by van der Waals forces. Within
each period a layer of molybdenum atoms are sandwiched
between two layers of sulphur atoms.
6
When the layer number is
reduced from multilayer to 1L, the band structure of MoS
2
transits from indirect (band gap 1.1 eV)
7
to direct band (band
gap 2.0 eV). Large band gap and high exciton binding energy
(600 meV)
8
of 1L-MoS
2
result into strong light emission in red
light (620–680 nm) even at room temperature.
9
However, in
contrast to other widely used rare earth compounds,
10,11
the PL
quantum efficiency of pristine 1L-MoS
2
is too low (0.4%)
6
to
meet the requirements of light emitting devices.
10,11
Hence
various methods such as localized surface plasmon (LSP)
12
resonance and molecule doping
13–15
etc., had been proposed to
enhance the PL intensity and tune the band position. Doping
using p-type molecules, such as H
2
O,
15
oxygen,
14,15
adzo-
benzene,
16
etc., allows to enhance the PL intensity of 1L-MoS
2
by
up to tens of times. Theoretically, the PL enhancement using
these p-type molecule is attributed to the weakened the Auger-
related non-radiative recombination, which is induced by the
reduced electron concentration of 1L-MoS
2
with the presence of
p-type dopants.
15
However, although H
2
O and oxygen molecules
are p-type molecules,
14,15
adsorbtion of such molecules from
ambient condition is unable to enhance the PL intensity of 1L-
MoS
2
.
14
Therefore activation process like vacuum annealing is
critical to enhance PL of 1L-MoS
2
with such molecules.
14,15
Using a p-type molecule with strong oxidability or good contact
with MoS
2
, such as tetracyanoquino-dimethane TCNQ,
13
such
activation process may be unnecessary and the preparation
process of 1L-MoS
2
with high PL yield can be greatly simplied.
With stronger oxidability than H
2
O and oxygen, H
2
O
2
can be a
useful p-type dopant that may effectively enhances the light
emission of 1L-MoS
2
without activation process. However, to
our best knowledge, few reports concerned on this topic had
been published yet.
In order to obtain effective PL enhancement, the dose of
H
2
O
2
molecules needs to be carefully controlled due to high
reactivity of H
2
O
2
molecules. If the concentration of H
2
O
2
is
high enough to react with MoS
2
following the equation MoS
2
+
7H
2
O
2
¼ MoO
3
+7H
2
O + 2SO
2
, the lattice of 1L-MoS
2
may
partially or totally react into MoO
3
and consequently the PL of
1L-MoS
2
is quenched. Therefore, low concentration of H
2
O
2
molecules is desirable to maintain intact lattice of 1L-MoS
2
so
that PL enhancement can be obtained. In this study, we
employed two different methods, i.e. physisorption of H
2
O
2
molecules on SiO
2
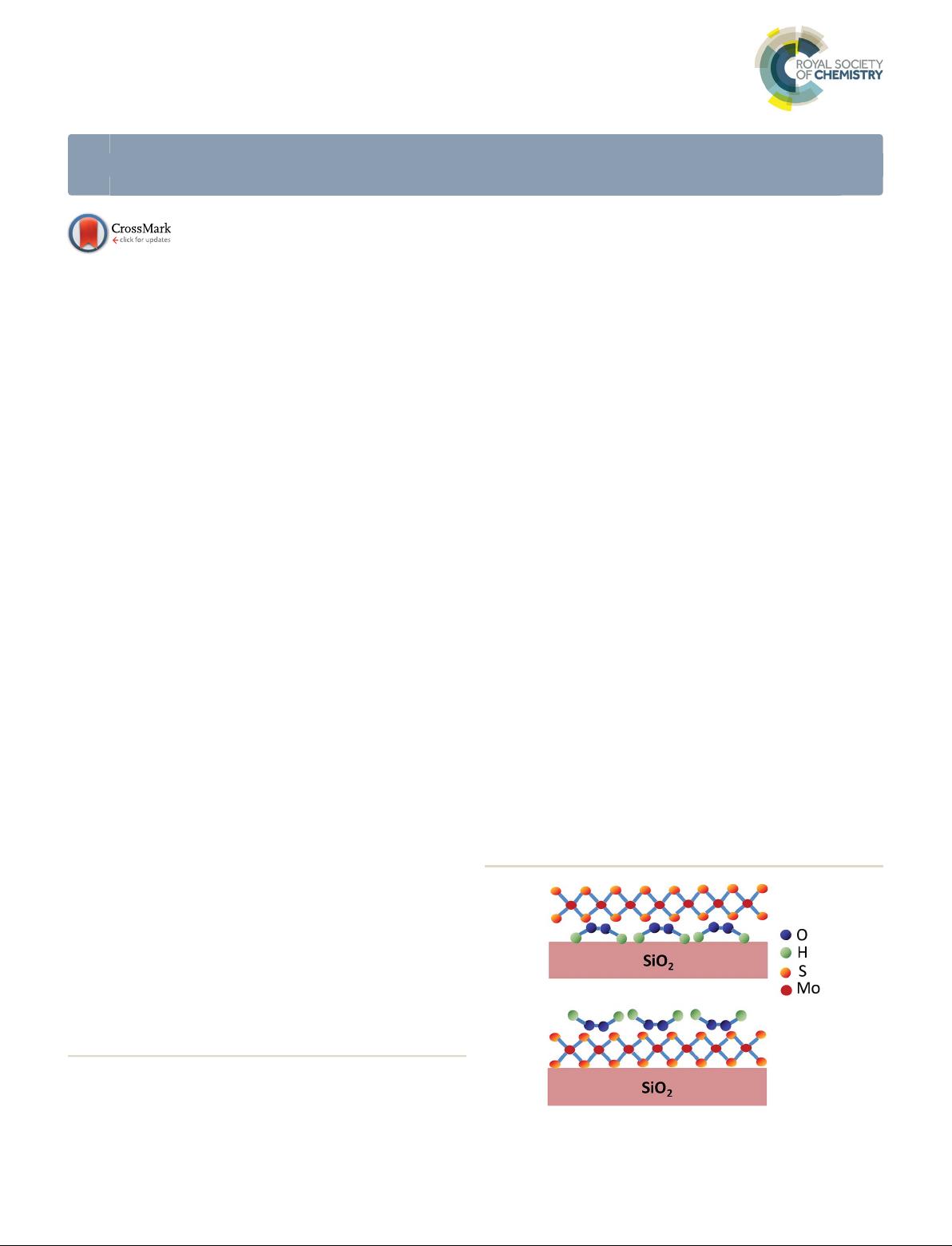
substrates (method I of Fig. 1) and spin-
Fig. 1 Schematic of H
2
O
2
doping 1L-MoS
2
prepared by method I (top
panel) and method II (bottom panel).
a
Institute of Materials Physics, Hangzhou Dianzi University, 310018, Hangzhou,
China. E-mail: suweitao@hdu.edu.cn; dxhuo@hdu.edu.cn; Fax: +86-571-86878539;
Tel: +86-571-86878539
b
Ningbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences,
315201, Ningbo, China
c
Department of Chemistry, Xi'an Jiaotong-Liverpool University, 215123, Suzhou, China
Cite this: RSC Adv.,2015,5, 82924
Received 27th June 2015
Accepted 24th September 2015
DOI: 10.1039/c5ra12450f
www.rsc.org/advances
82924 | RSC Adv.,2015,5, 82924–82929 This journal is © The Royal Society of Chemistry 2015
RSC Advances
PAPER









