Different ways to make a head
Urs Schmidt-Ott
Summary
In
Drosophila
, the establishment of the larval head and
thorax depends on the transcription factors BICOID and
HUNCHBACK, and on signalling mediated by the receptor
tyrosine kinase TORSO. Genetic experiments described
in two recent papers
(1,2)
demonstrate that these factors
can, to a large extent, replace each other, revealing a
surprising degree of plasticity in establishing larval
anterior structures. The commutability of developmental
factors might in part reflect the evolutionary history of the
system. BioEssays 23:8±11, 2001.
ß 2001 John Wiley & Sons, Inc.
Introduction
Insect embryos develop in response to factors spreading from
the poles of the patterning system. Although the experimental
findings that suggested this general principle in the premole-
cular era came from insects as diverse as leafhopper, beetles,
midges and flies,
(3±5)
the proposed anterior determinant has
since been substantiated only in some flies, where it is
encoded by the bicoid gene.
(6±9)
Anteriorly localized maternal
bicoid mRNA encodes a homeodomain protein (BICOID) that
specifies anterior development in a concentration-dependent
manner through spatially restricted activation of genes re-
quired for segmentation.
(10±12)
BICOID enhances the expres-
sion of the transcription factor HUNCHBACK in the anterior
half of the Drosophila embryo.
(13±15)
Both proteins share in
activating target genes involved in the specification of distinct
portions of the head and thorax.
(16)
BICOID-dependent gene
activation in the most anterior portion of the head is not
enhanced by HUNCHBACK but by a signalling pathway
(reviewed in Ref. 17), which is dependent on the tyrosine-
kinase-receptor TORSO
(18±20)
(Fig. 1). Two recent papers
describe genetic experiments that reveal that BICOID can
substitute for TORSO-mediated signalling activity in the head
and that HUNCHBACK can partially substitute for BICOID.
(1,2)
Thorax formation in the absence of
BICOID activity
Wimmer et al.
(1)
asked to what extent HUNCHBACK activity
requires BICOID function. Three hunchback enhancers are
known: one maternal, one early zygotic, and one late
zygotic.
(14)
Only the early zygotic enhancer/promotor is
BICOID-dependent.
(11,12,21,22)
How do flies deprived of the
BICOID-dependent enhancer develop? To address this
question hunchback rescue constructs without the BICOID-
dependent early zygotic enhancer element were engineered
(hbP1only) and introduced into flies. Embryos deficient for
early zygotic hunchback activity lack all three thoracic and
the labial segments, if derived from heterozygous hunchback
mutant mothers (Fig. 2A). However, if the full maternal
contribution of HUNCHBACK to the embryos is restored by
introducing a BICOID-independent copy of the hunchback
gene into the mother, the first thoracic and the labial segments
are rescued (Fig. 2B). A complete rescue is observed, if mate-
rnal hunchback is further increased to four copies and knirps,
coding for a zygotic transcription factor with a repressive effect
on hunchback, is lowered from two copies to one (Fig. 2C).
These experiments demonstrate that, under certain condi-
tions, natural BICOID-independent hunchback enhancers can
restore essential hunchback expression sufficient to cause
thoracic and labial segments.
Is this effect of HUNCHBACK due to replacing BICOID
function? This question was addressed in a second series of
experiments. BICOID-deficient embryos lack the entire head
and thorax, which are replaced by posterior abdominal
structures in reversed polarity. When such embryos, which
show normal maternal hunchback expression, were provided
with zygotic HUNCHBACK activity in form of a BICOID-like
anterior HUNCHBACK gradient, the duplicated posterior
structures at the anterior pole of the embryos were almost
completely suppressed. In addition, two of the three thoracic
segments were rescued. Thus, an increased amount of
HUNCHBACK can partially replace BICOID function in the
thorax.
8 BioEssays 23.1 BioEssays 23:8±11, ß 2001 John Wiley & Sons, Inc.
Max-Planck-Institut fu
È
r biophysikalische Chemie, Abteilung fu
È
r mole-
kulare Entwicklungsbiologie, Am Fassberg 11, D-37077 Go
È
ttingen,
Germany. E-mail: uschmid@gwdg.de
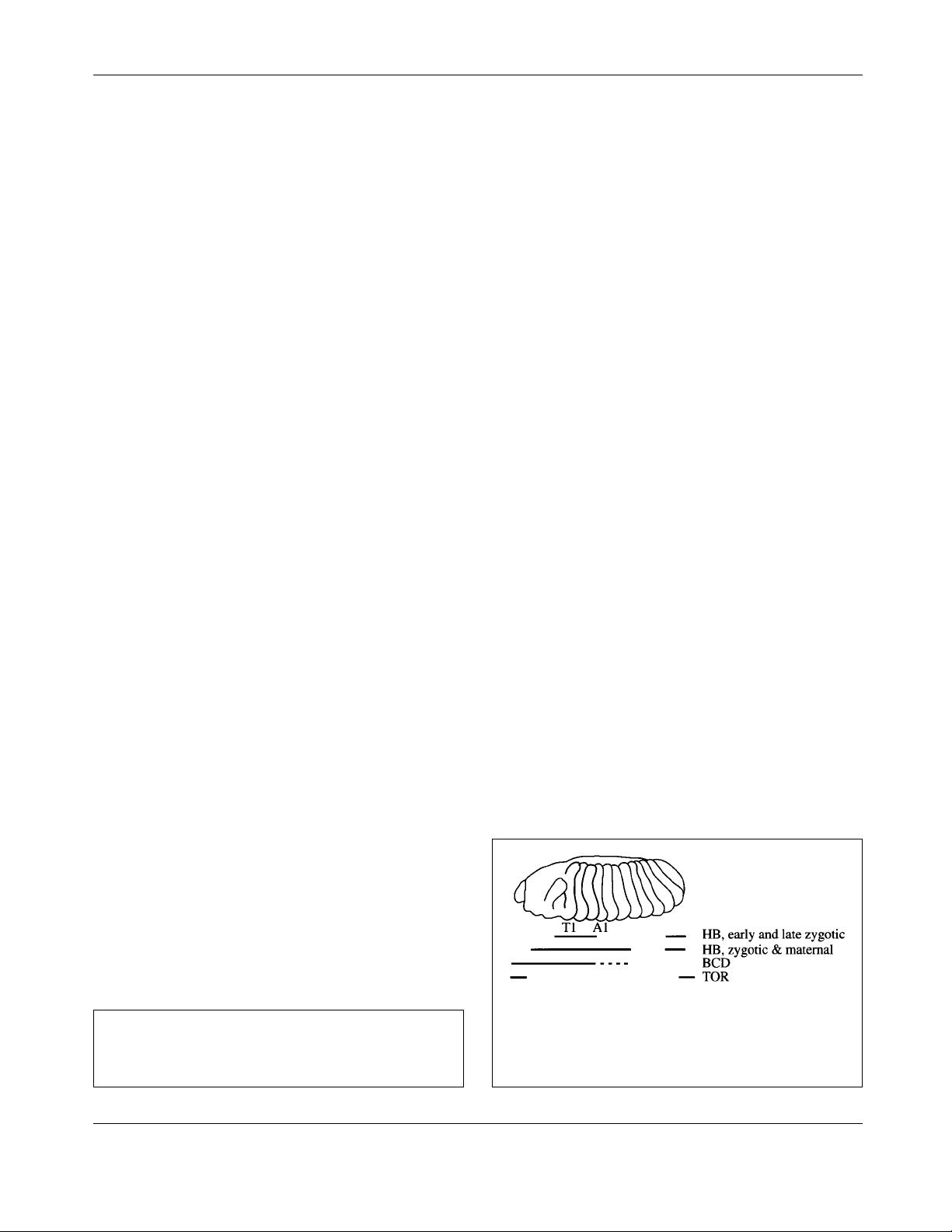
Figure 1. Schematic view of a segmented embryo. Seg-
mental deletions dependent on BICOID (BCD), HUNCHBACK
(HB) and TORSO (TOR)-mediated signalling are indicated
with bars. T1 and A1 mark the first thoracic and the first
abdominal segments, respectively.
What the papers say















































