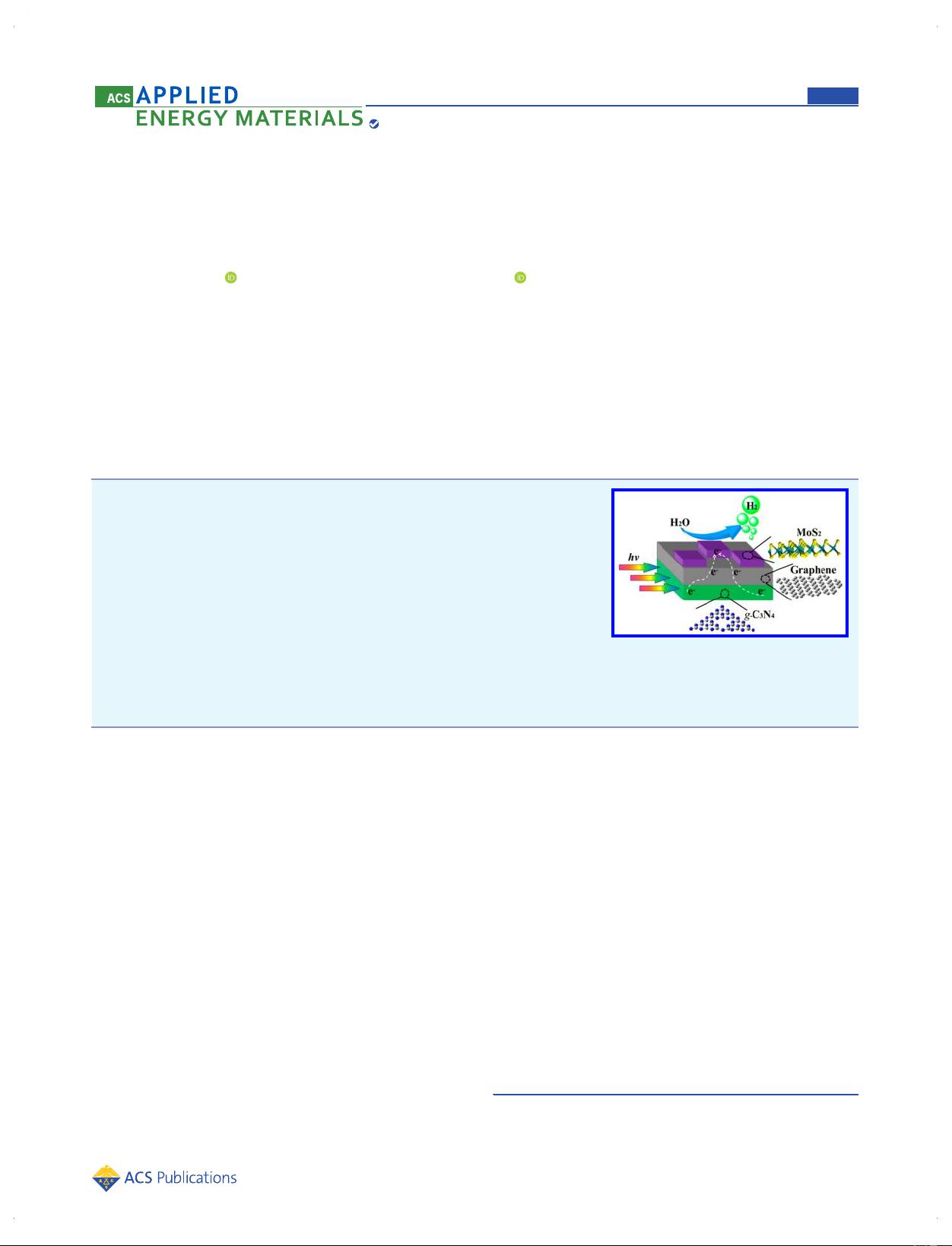
Promoting Charge Separation in g‑C
3
N
4
/Graphene/MoS
2
Photocatalysts by Two-Dimensional Nanojunction for Enhanced
Photocatalytic H
2
Production
Yong-Jun Yuan,*
,†
Yan Yang,
†
Zijian Li,
†
Daqin Chen,*
,†
Shiting Wu,
†
Gaoliang Fang,
†
Wangfeng Bai,
†
Mingye Ding,
†
Ling-Xia Yang,
‡
Da-Peng Cao,
§
Zhen-Tao Yu,*
,‡
and Zhi-Gang Zou
‡,∥
†
College of Materials and Environmental Engineering, Hangzhou Dianzi University, Hangzhou 310018, People’s Republic of China
‡
National Laboratory of Solid State Microstructures and Collaborative Innovation Center of Advanced Microstructures, College of
Engineering and Applied Science, Nanjing University, Nanjing 210093, People’s Republic of China
§
College of Materials Science and Engineering, Nanjing University of Posts and Telecommunications, Nanjing 210023, People’s
Republic of China
∥
Macau Institute of Systems Engineering, Macau University of Science and Technology, Macau 999078, People’s Republic of China
*
S
Supporting Information
ABSTRACT: Graphitic carbon nitride (g-C
3
N
4
) is a promising photocatalyst for solar
H
2
generation, but the practical application of g-C
3
N
4
is still limited by the low
separation efficiency of photogenerated charge carriers. Herein, we report the
construction of ternary g-C
3
N
4
/graphene/MoS
2
two-dimensional nanojunction
photocatalysts for enhanced visible light photocatalytic H
2
production from water.
As demonstrated by photoluminescence and transient photocurrent studies, the
intimate two-dimensional nanojuction can efficiently accelerate the charge transfer,
resulting in the high photocatalytic activity. The g-C
3
N
4
/graphene/MoS
2
composite
with 0.5% graphene and 1.2% MoS
2
achieves a high H
2
evolution rate of 317 μmol h
−1
g
−1
, and the apparent quantum yield reaches 3.4% at 420 nm. More importantly, the
ternary g-C
3
N
4
/graphene/MoS
2
two-dimensional nanojunction photocatalyst exhibits much higher photocatalytic activity than
the optimized Pt-loaded g-C
3
N
4
photocatalyst.
KEYWORDS: interface engineering, artificial photosynthesis, two-dimensional nanojunction, hydrogen production, carrier separation
T
he sunlight driven generation of the carbon-free H
2
from
water splitting using semiconductor photocatalysts
represents a promising strategy to convert solar energy to
chemical energy for sustainable development.
1−3
Since the
pioneering work of photolysis of water on TiO
2
electrodes by
Fujishima and Honda,
4
numerous semiconductors have been
designed and developed as photocatalysts for photocatalytic H
2
production from water during the past 40 years, such as TiO
2
,
5
Cu
2
O,
6
SrTiO
3
,
7
CdS,
8 ,9
ZnIn
2
S
4
,
10
CuInS
2
,
11
CdSe,
12
ZnO:GaN,
13
and g-C
3
N, etc.
14
Among these different semi-
conductors, graphitic carbon nitride (g-C
3
N
4
) is one of the
most promising photocatalysts for visible light photocatalytic
H
2
generation owing to its relatively narrow band gap of 2.8 eV,
suitable band edge positions, cost effectiveness, low toxicity, as
well as excellent durability.
15−18
However, the intrinsic property
of rapid recombination of photogenerated electron−hole pairs
limits the photocatalytic activity of g-C
3
N
4
for H
2
generation.
15
An efficient strategy to overcome the above drawback is to load
a suitable cocatalyst on g-C
3
N
4
to provide abundant active sites,
which could not only accelerate the transfer and migration of
photgenerated charge carriers, but also lower the activation
potential for the H
2
evolution reaction. Especially, noble metals
such as Pt,
19
Pd,
20
and Au, etc.,
21
can act as e fficient cocatalysts
to enhance the photocatalytic performance of g-C
3
N
4
.
However, noble metals are rare and expensive; thus, it is of
great significance to develop noble-metal-free cocatalysts.
Recently, numerous low-cost transition-metal-based cocata-
lysts including molybdenum, nickel, and cobalt compounds
have been developed for g-C
3
N
4
-based photocatalytic hydrogen
production systems.
22−29
Among these transition-metal com-
pounds, MoS
2
has been proven to be an efficient cocatalyst
owing to abundant exposed edges and low overpotential for a
H
2
evolution reaction.
25
Unfortunately, the poor electrical
conductivity of MoS
2
restricts its catalytic activity. Recent
studies showed that the electrical conductivity and catalytic
activity of MoS
2
can be improved by coupling MoS
2
with
gra phene due to the superior charge transfer ability of
graphene.
30−33
More importantly, both MoS
2
and graphene
have the same layered structure as g-C
3
N
4
, which achieves a
well-defined two-dimensional (2D) junction. The construction
of ternary g-C
3
N
4
/RGO/MoS
2
photocatalysts can provide large
and intimate two-dimensional nanojunctions, which would play
a key factor in determining the charge separation efficiency and
Received: January 8, 2018
Accepted: March 13, 2018
Published: March 13, 2018
Letter
www.acsaem.org
Cite This: ACS Appl. Energy Mater. 2018, 1, 1400−1407
© 2018 American Chemical Society 1400 DOI: 10.1021/acsaem.8b00030
ACS Appl. Energy Mater. 2018, 1, 1400−1407
Downloaded via NANJING UNIV POSTS & TELECOM on October 12, 2018 at 08:52:11 (UTC).
See https://pubs.acs.org/sharingguidelines for options on how to legitimately share published articles.









