fluorophore products, and the excess fluorescamine can be hydrolyzed into non-
fluorescent products very fast. By measuring the emission band centered at
470 nm (
l
ex
¼ 390 nm), the presence of amino group can be validated.
2.6. Surface modification of the nanoparticles with FA and PEG
To covalently conjugate 2, 9, 16, 23-tetracarboxylic Zinc phthalocyanine and folic
acid on UCNPs, 5 ml of dimethylformamide solution containing 0.8 mg of 2, 9, 16, 23-
tetracarboxylic Zinc phthalocyanine, 0.5 mg of folic acid, 1 mg of 1-ethyl-3- (3-
dimethylaminopropyl) carbodiimide and 1 mg of N-hydroxy-succinimide were
incubated at room temperature for 2 h, and then 10 mg of amino-functionalized
NaYF
4
:Yb
3þ
,Er
3þ
upconversion nanoparticles was added into the solution and
stirred vigorously for 24 h. The mixture was then centrifuged at 10,000 rpm for
10 min three times to spin down the nanoparticles. The supernatant was withdrawn
carefully and the particles resuspended in DMSO and ethanol mixture solution
(DMSO: ethanol ¼ 4:1) for further used. To reduce the undesired toxicity of nano-
particles to normal tissue, the surface of UCNPs-ZnPc/FA were coated with PEG. 4 ml
of DMSO and ethanol mixture solution containing 1 mg PEG-SC and 1 mg UCNPs-
ZnPc/FA was shaken by shaking table. The mixture was then centrifuged at
10,000 rpm for 10 min to spin down the nanoparticles. The supernatant was
withdrawn carefully and the particles resuspended in PBS (PH ¼ 7.4). The washing
process was repeated twice.
2.7. To assess the NIR exposure effect on the cells
Hela cells that over express folate receptors and A549 that have a low expression
of folate receptors were purchased from First Bethune Hospital, University of Jilin.
HeLa cells were grown in a 96-well cell-culture plate at 10
4
e10
5
per well and then
incubated for 24 h at 37
C under 5% CO
2
. A power adjustable 980 nm fiber laser with
maximal output power of 30 W (n-LIGHT Corporation) was collimated and
employed as area light source to irradiate the 96-well plate. After 10 min exposure of
980 nm light at different power density (0, 0.39, 0.85, 1.23, 1.61, 1.99 W/cm
2
), the
cells were allowed to incubate for an additional 48 h. To assess the effect of exposure
to NIR laser on the cells, the cell viability was measured by 3-(4, 5-dimethylthiazol-
2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay.
2.8. Targeted cancer cell imaging and therapy
Hela cells cultured in folate-free RPMI-1640 medium was considered as the
positive group, while HeLa cells cultured in normal RPMI-1640 medium and A549
cells which were cultured in folate-free RPMI-1640 medium were considered as a
negative group. All the mediums were supplemented with 10% fetal bovine serum,
100
m
g/mL penicillin, and 100
m
g/mL streptomycin. Cells were cultivated in medium
at 37
C in a humidified 95% air and 5% carbon dioxide (CO
2
) atmosphere. The
cytotoxicity was measured using a standard MTT assay. HeLa cells were grown in a
96-well cell-culture plate at 10
4
e10
5
per well and then incubated for 24 h at 37
C
under 5% CO
2
. After 24 h culturing, UCNPs-ZnPc/FA nanoconjugates were added to
the culture medium at different concentrations, with five parallel wells for each
concentration (0, 100, 200, 400, 800
m
g/mL). The standard MTT assay was carried out
to determine the cell viabilities relative to the untreated cells. For the FA targeted
PDT experiment, the cells were allowed to incubate with different concentration
UCNPs-ZnPc/FA (0, 100, 200, 400, 800
m
g/mL) for another 24 h at 37
C and then
washed twice with PBS before being exposed to NIR irradiation. A power adjustable
980 nm fiber laser with maximal output power of 30 W was collimated and
employed as area light source to irradiate the 96-well plate. After 10 min exposure of
980 nm light at 0.39 W/cm
2
, the cells were allowed to incubate for an additional
48 h. To assess the effect of exposure to NIR laser on the cells with and without the
nanoparticles, the cell viability was measured by MTT assay. Typically, 10
m
L of MTT
solution (5 mg/mL MTT in PBS) was added to each well and incubated for 4 h at
37
C. After removing the medium, the wells were washed by PBS, and then the
intracellular formazan crystals were extracted into 100
m
l of DMSO. The absorbance
of cell lysate was recorded at 490 nm by a microtiter plate reader, and the cell
viability could be calculated from the average value of four parallel wells.
2.9. Upconversion luminescent imaging of cells
For the FA targeted imaging experiment, both positive and negative cells were
seeded in the confocal dishes at a concentration of 10
3
/ml. After 24 h of cell
attachment, both positive and negative cells were incubated with 200
m
g/mL UCNPs-
ZnPc/FA for 2 h at 37
C. Before imaging all cells were washed three times and fixed
in 4% paraformaldehyde for 20 min at RT. Cells were washed twice with PBS three
times. The nuclei were then counterstained with 0.1
m
g/mL DAPI for 10 min at RT
followed by twice washing with PBS for three times. Upconversion fluorescence
imaging were then performed using a Nikon Inverted Microscope Eclipse Ti-U Main
Body (Nikon, Tokyo, Japan) equipped with C2-SHS Scanner and Controller under
excitation of lower power density (0.19 W/cm
2
).
2.10. In vivo PDT
Female C57/6J mice (20 g, 6e 8 weeks old) used in this study were purchased
from First Bethune Hospital, University of Jilin. All experiments were carried out in
compliance with the animal management. The Hepa1-6 tumor model was estab-
lished by subcutaneously inoculating Hepa1-6 cells (3 10
6
) into the upper axillary
fossa in the mice (n ¼ 6). The mice were investigated when the tumor grew to a
diameter of 4e6 mm. 100
m
l saline or UCNPs-ZnPc/FA (10 mg/ml) was intratumorally
injected into each Hepa1-6 tumor-bearing mouse. The mice were randomly assigned
into four groups treated with different injections, as follows: (1) group 1 received
only intratumoral injection of the saline (the control group, n ¼ 6); (2) group 2
received intratumoral injection of the saline with NIR light irradiation (n ¼ 6); (3)
group 3 received intratumoral injection of the UCNPs-ZnPc/FA (n ¼ 6); (4) group 4
received intratumoral injection of the UCNPs-ZnPc/FA with NIR light irradiation
(n ¼ 6). The tumors were irradiated with a 980 nm laser light (0.39 W cm
2
) for
15 min. To avoid any tissue damage by heating, the laser treatment was done with
3 min interval for every 3 min of light exposure. After treatment, the tumor volume
was calculated as length (width)
2
1/2 with a caliper over 2 weeks. The body
weight of each mouse was monitored every other day over 2 weeks. Relative tumor
volume, relative body weight and inhibition ratio were defined as follows: Relative
tumor volume ¼ V/V
0
, V
0
and V stand for the tumor volume on the initial day and on
the day of measurement, respectively. Relative body weight ¼ W/W
0
, W
0
and W are
the body weight of mouse on the initial day and on the day of measurement,
respectively. Inhibition ratio ¼ (Vc Vt)/Vc 100%, Vt and Vc represent the average
tumor volume for the control group and treatment group, respectively.
2.11. Histology examination
Histology analysis was performed at the 14th day after treatment. The organs
(heart, liver, spleen,lungand kidney) and tumor tissuesof the mice in the control group
and treatment group were isolated from the mice, fixed with 10% neutral buffered
formalin and embedded in paraffin. The sliced organs and tumor tissues (8 mm) were
stained with Hematoxylin and Eosin (H&E) and examined by a microscope.
3. Results and discussion
3.1. Synthesis of UCNPs and covalently constructing UCNPs-ZnPc/FA
nanophotosensitizer
The NaYF
4
: 25% Yb
3þ
, 0.2% Er
3þ
oleic acid coated UCNPs were
used as the donor, instead of 20% Yb
3þ
used generally in the current
studies to increase the upconversion luminescence (UCL) at 660 nm
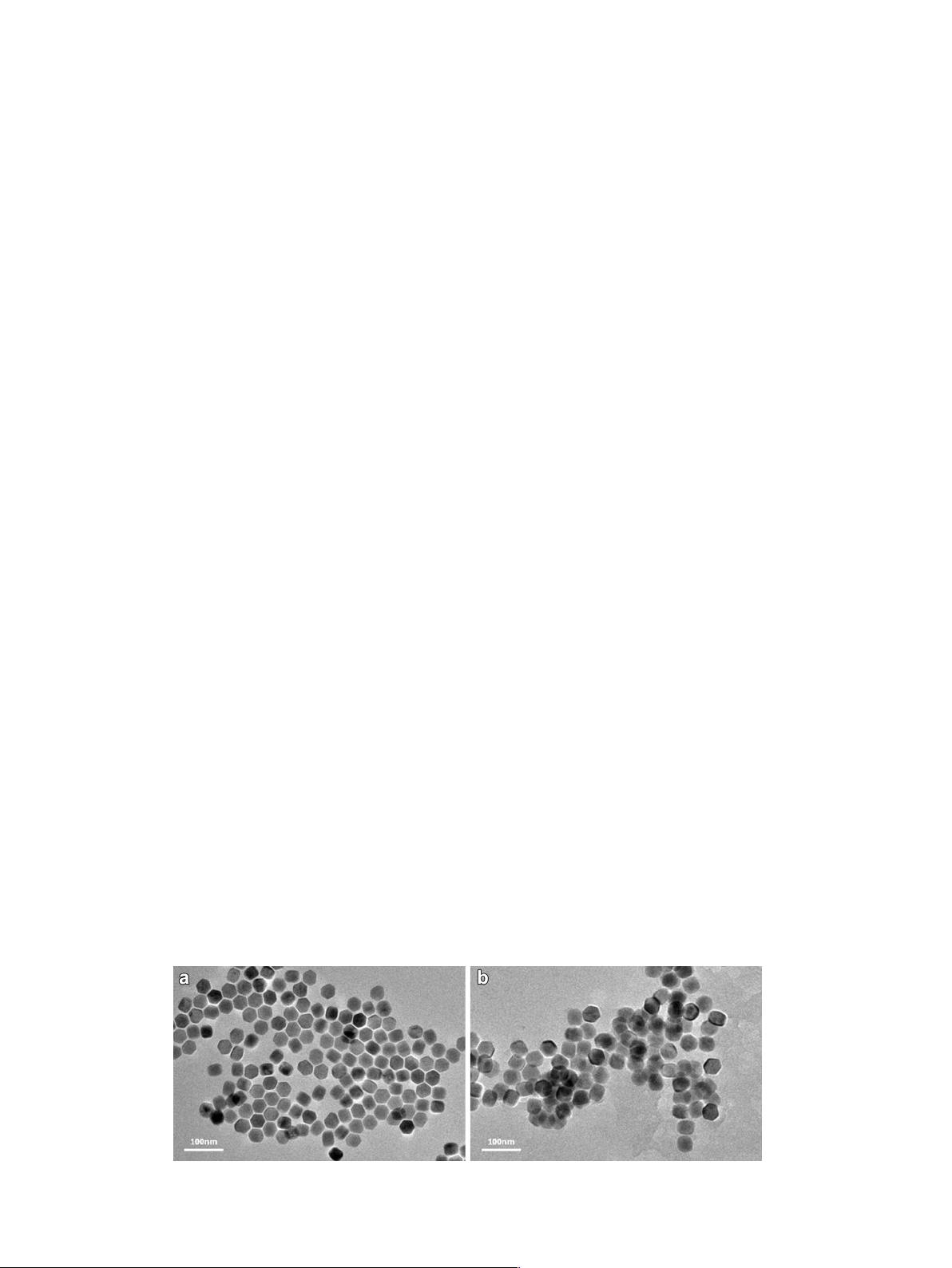
(Fig. S1) and their morphologies and phase purities were analyzed
by TEM and XRD, respectively, as shown in Fig. 1 and Fig. S2. Good
Fig. 1. TEM image of NaYF
4
:Yb
3þ
,Er
3þ
UCNPs (a) before phase transfer and (b) after phase transfer.
L. Xia et al. / Biomaterials 35 (2014) 4146e41564148



































