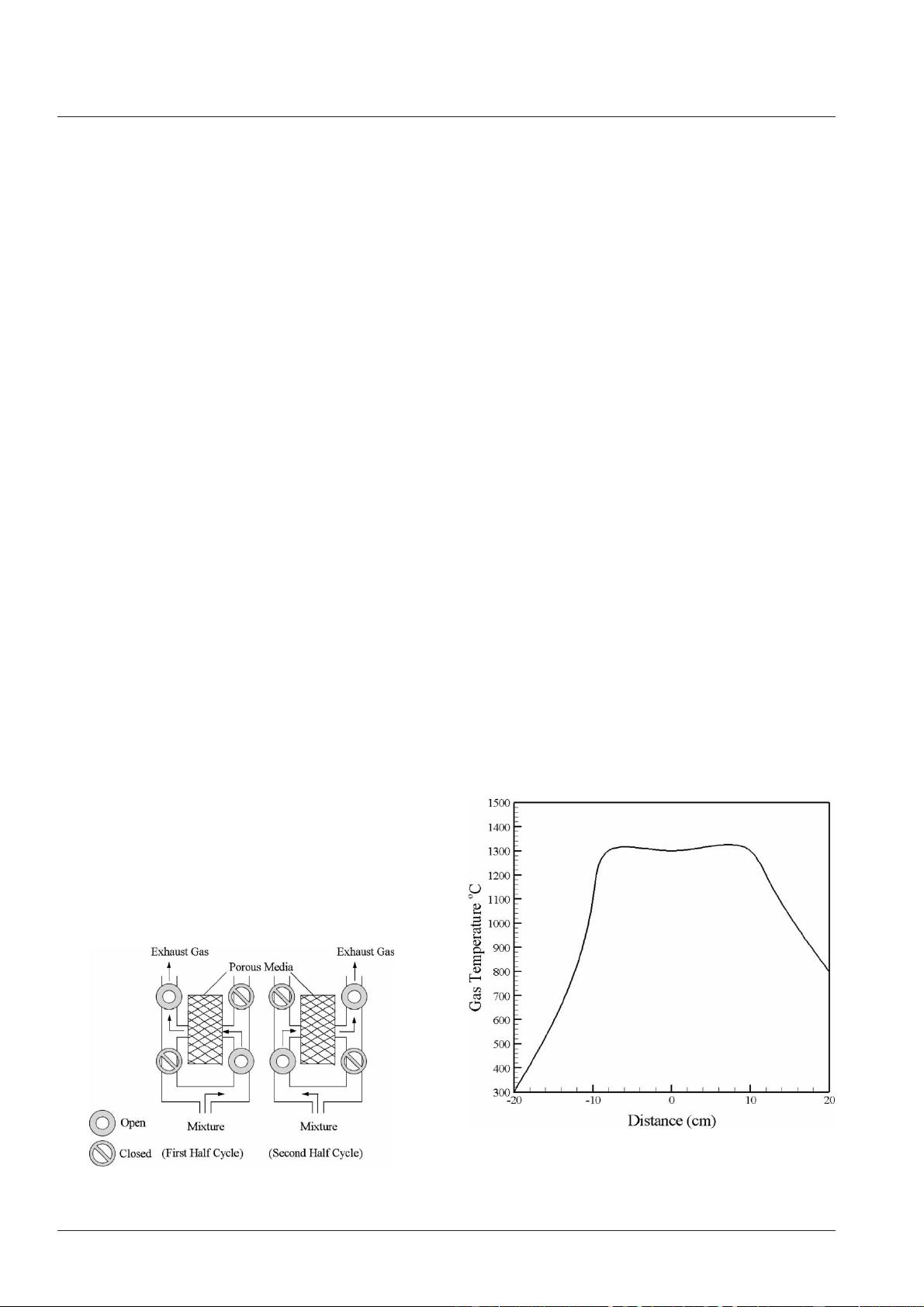
profiles exhibit a maximum at the exit side of the
flow. Thereafter, the flow direction is altered to the
opposite side by the aid of four valves (Fig. 3). On
the reverse flow half-cycle, the fresh combustibles
are encountering much higher solid temperatures
at the entering side (which was highly heated by
the combustion products on the previous half-
cycle). Consequently, the amount of heat recycled
becomes larger than that with the single flow direc-
tion and hence the degree of excess enthalpy is
higher. In such a case, after combustion releases
the energy, the gas temperatures on the reverse
half-cycle will continue to rise towards the exit side
(which was the entrance side in the previous half-
cycle) and the temperature maximum region will
be shifted towards that exit side with a subsequent
broadening in the solid heated length. When the per-
iodic time for the flow switch is properly adjusted
and a steady-state condition is reached, the tempera-
ture profiles will exhibit a trapezoidal shape (Fig. 4).
This mechanism cannot be produced by single flow
direction, as the direction of solid heat transfer is
from higher to lower solid temperatures, which
then settles a single gradient for both sol id and gas
temperature profiles. Conversely, in the reciprocat-
ing flow system, the heat transfer from the combus-
tion gases raises the solid temperatures from both
directions. This consequently leads to the trapezoi-
dal shape and the higher temperature rise from
both sides with a net gas temperature increase at
the exit (Fig. 4). At the optimum flow reversal peri-
odic time, the maximum temperature rise in the
porous medium excee ded the theoretical one by a
factor of about 2.2. Extinction occurred by lowering
the heat transfer coefficient, the thermal conduc-
tivity, or the heat capacity of the solid relative to
the gas. Hoffmann et al. [38] analysed the develop-
ment of temperature profiles in the porous solid
during a half-cycle. By increasing the half-cycle
time, the temperature profile turned to a triangular
shape and a further increase led to flame extinction.
3.3 Utilization of porous burners for synthetic gas
production
Similar to the extension in the lean operation equiv-
alence ratio for combustion in porous media by the
preheating effect, there is also an extension in the
upper flammability limit for rich gaseous mixtures.
This feature is utilized for production of syngas
(CO þ H
2
) by burning hydrocarbon fuels in a
deficient supply of air, so that the following partial
oxidation may take place
2C
m
H
n
þ mO
2
! 2mCO þ nH
2
(1)
Because the rates of elementary reactions leading
to the above overall reaction are low at the adiabatic
flame temperatures of rich mixtures (below 1000 8C),
the insertion of porous media provides the internal
heat recirculation necessary to sustain the partial
oxidation. Therefore, super-adiabatic temperatures
are obtained and reactions are drive n to produce
H
2
and CO. The necessity in compact and efficient
hydrogen source for fuel-cell-powered vehicles
stimulates studies to develop technologies for
reforming hydrocarbon fuels. For such development,
numerous works have been performed to investigate
the rich gas mixture combustion in porous burners.
The major parameters that affect reaction rates in
these burners were found to be the maximum tempe-
rature and residence time, as reported by Bingue
et al. [39], Drayton et al. [40], and Kennedy et al.
[41]. In the work of Pedersen-Mjaanes et al. [42], a
stationary flame could be stabilized in an insulated
porous burner. The hydrogen production was
Fig. 4 Steady-state gas temperature profile for a half-
cycle inside reciprocating flow porous
medium burner [35]
Fig. 3 Reciprocating flow in a porous medium burner
490 M M Kamal and A A Mohamad
Proc. IMechE Vol. 220 Part A: J. Power and Energy JPE169
#
IMechE 2006
at University of Victoria on August 30, 2012pia.sagepub.comDownloaded from









