3D retinal SD-OCT images. This is due to two main reasons.
First, the structure of the retina is different from that of the
brain. Geometric moment invariants (GMIs) can distinguish
different geometric structures of brain, but it fails to distinguish
the plate-like structure of retinal layers. Second, retinal
SD-OCT images have much higher resolution than brain MRI
images. In HAMMER, the driving voxels are selected by using
a fuzzy clustering method. Since the fuzzy clustering method
needs to calculate the distance between voxels, it is time
consuming especially when the data is large and may have local
minima. Our work is inspired by HAMMER and tries to extend
HAMMER to OCT registration. Compared with other retinal
OCT image registration methods, we adopt HAMMER's
hierarchical attribute matching mechanism to improve the
registration accuracy while reducing the computation
complexity. Furthermore, our work presents several novel
elements compared with HAMMER: 1)We propose to use
intensity-based region feature, surface-based structure feature
and vessel-like feature instead of GMI feature to distinguish
different structures of retina. 2) We propose an efficient
driving voxel selection method to further reduce the
computation complexity. In our method, rather than randomly
selecting the active voxels, the active voxels are hierarchically
selected following a strategy for importance coefficient
assignment. 3) A preprocessing step is designed to remove the
motion distortion in retinal OCT data before registration. To the
best of our knowledge, the proposed method is the first
longitudinal retinal OCT image registration algorithm which
considered both normal data and serious pathological data.
III. METHODS
A. Overview of the Approach
The deformable transformation is a free form mapping at
each voxel
. It can be solved by finding a transformation of
each voxel such that the energy function is minimized.
Considering the high resolution of OCT data, the energy
function would be a very high dimension function which makes
it extremely difficult to find the global optimal solution. The
main difficulties are the computation complexity and the local
minima problem. To speed up registration process and reduce
local minima, a novel design-detection-deformation
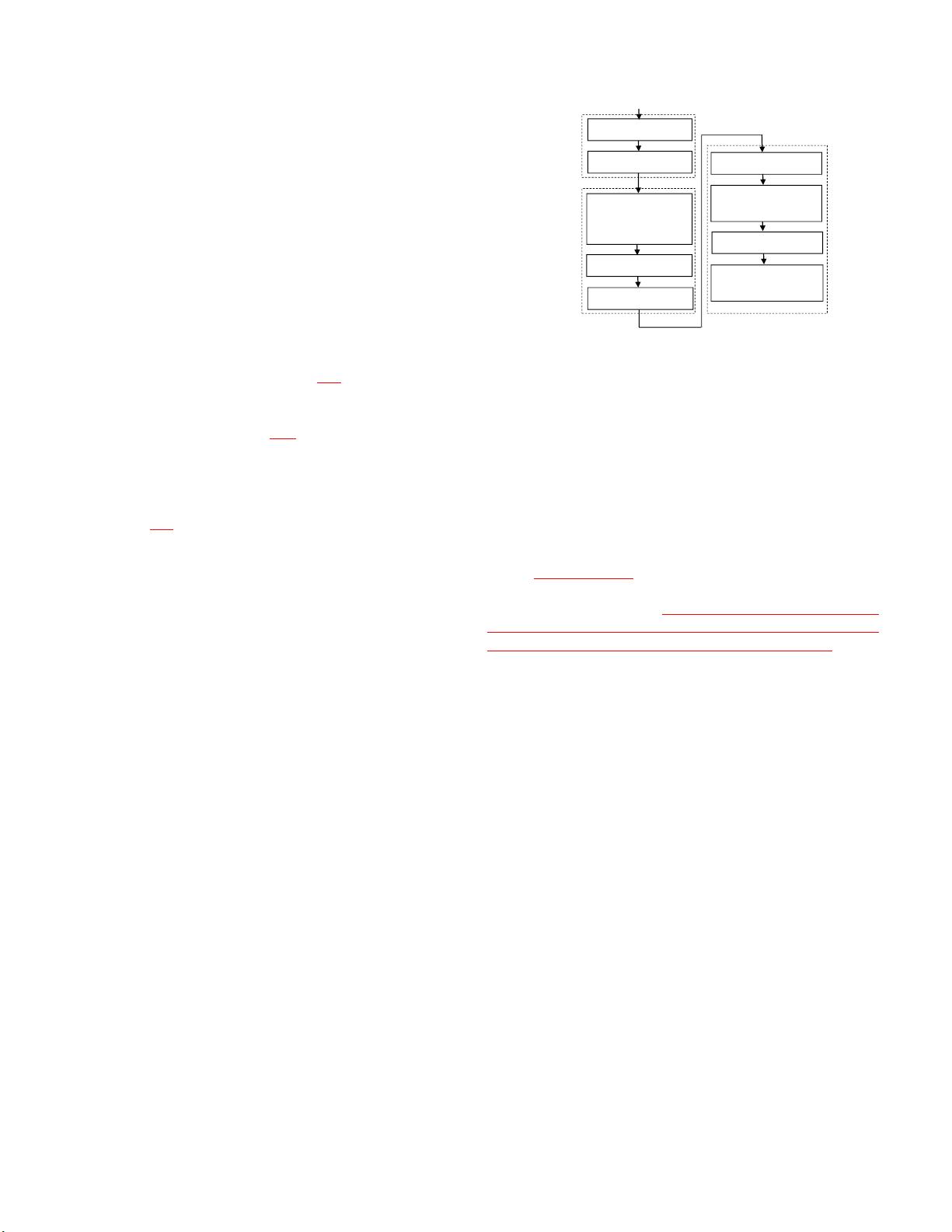
mechanism is designed. The proposed method consists of four
steps: preprocessing, feature design, correspondence detection
and hierarchical deformation. The overall flowchart is shown in
Fig. 2. In preprocessing step, OCT data are first segmented by
detecting 7 surfaces using graph search-based method and then
B-scans are flattened to correct eye movement. In the design
step, a couple of features are designed for each voxel in the
image. In the detection and deformation step, active voxels are
hierarchically selected and point-to-point correspondences
between the subject and the template images are established.
The image is then hierarchically deformed according to the
detected correspondences in multi-resolution. The detail of
each step is discussed in the following parts.
Input template and
subject image
Output 3D registration
result
Fig. 2. Flowchart of the proposed algorithm.
B. Preprocessing
1) Multi-resolution graph search: 3D graph-based optimal
surface segmentation method can detect multiple interacting
surfaces simultaneously [21]. The basic idea is to transform the
optimal surface detection problem into computing a minimum
cut in an arc-weighted directed graph. This method and its
variations are successfully applied to retinal layer segmentation
of macular optical coherence tomography images [22-31]. The
surface segmentation methods designed for normal retinas can
also be used to segment the retinas with glaucoma and multiple
sclerosis or other diseases without dramatic change in the layer
structure in the early stage. However, it is difficult to segment
the retinas with additional layer structures such as sub-RPE
fluid and intra-retinal cysts by using the same methods. In our
algorithm, by considering both normal and serious pathological
data such as CNV, the surface segmentation is conducted based
on our previously proposed constrained graph search method
[32]. In preprocessing, all B-scans of the template image and
the subject image are segmented by seven retinal surfaces as
shown in Fig.3. These seven surfaces partition an OCT dataset
into six layers: (1) retinal nerve fiber layer (RNFL), (2)
ganglion cell layer and inner plexiform layer (GCL+IPL), (3)
inner nuclear layer (INL), (4) outer plexiform layer (OPL), (5)
outer nuclear layer and inner/outer segment layer
(ONL+IS/OSL), (6) retinal pigment epithelium (RPE).
2) B-scans flattening: Eye movement artifact occurring
during 3D OCT scanning is a problem for retinal OCT imaging
and makes image registration difficult. During OCT acquisition
process, since the volume is acquired in a few seconds, eye
movement caused by heart beat and respiration in the scan
process results in motion artifacts. In motion distorted data, the
positions of layers varies greatly in consecutive B-scans which
make interpolation and regularization difficult. The position
shifts of B-scans can be viewed in the y-z slices, as shown in
Fig. 4 (a), where each column corresponds to a B-scan.
Flattening the 3D OCT volumes is often used to correct eye






















