12 2
SOIL FORMATION
velopment of Earth (Hattori, 1973; McCarty, 2004).
Photosynthetic bacteria, cyanobacteria, or ‘‘blue-green
bacteria’’ evolved about 3.5 billion years ago (Proter-
ozoic era—Precambrian), and they are the oldest
known fossils. Cyanobacteria use energy from the sun
to reduce the carbon in CO
2
to cellular carbon and to
obtain the needed electrons for oxidizing the oxygen
in water to molecular oxygen. During the Archaean
period (2.5 billion years ago), cyanobacteria converted
the atmosphere from reducing to oxidizing and
changed the mineral nature of Earth.
Eukaryotic algae evolved later, followed by the mul-
ticellular eukaryotes including plants. Photosynthesis
is the primary producer of the organic particulate mat-
ter in shale, sand, silt, and clay, as well as in coal,
petroleum, and methane deposits. Furthermore, cyano-
bacteria and algae increase the water pH when they
consume CO
2
dissolved in water, resulting in carbonate
formation and precipitation of magnesium and calcium
carbonates, leading to Earth’s major carbonate forma-
tions.
Aerobic bacteria live in the presence of dissolved
oxygen. Anaerobic bacteria survive only in the absence
of oxygen. Facultative bacteria can live with or without
oxygen. Some bacteria may resort to fermentation to
sustain their metabolism under anaerobic conditions
(Purves et al., 1997). For example, in the case of an-
aerobic conditions, fermenting bacteria oxidize carbo-
hydrates to produce simple organic acids and H
2
that
are used to reduction of ferric (Fe
3
⫹
) iron, sulfate re-
duction, and the generation of methane (Chapelle,
2001). Microbial energy metabolism involves electron
transfers, and the electron sources and acceptors can
be both organic and inorganic compounds (Horn and
Meike, 1995). Most soil bacteria derive their carbon
and energy directly from organic matter and its oxi-
dation. Some other bacteria derive their energy from
oxidation of inorganic substances such as ammonium,
sulfur, and iron and most of their carbon from carbon
dioxide. Therefore, biological activity mediates geo-
chemical reactions, causing them to proceed at rates
that are sometimes orders of magnitude more rapid
than would be predicted solely on the basis of the ther-
mochemical reactions involved.
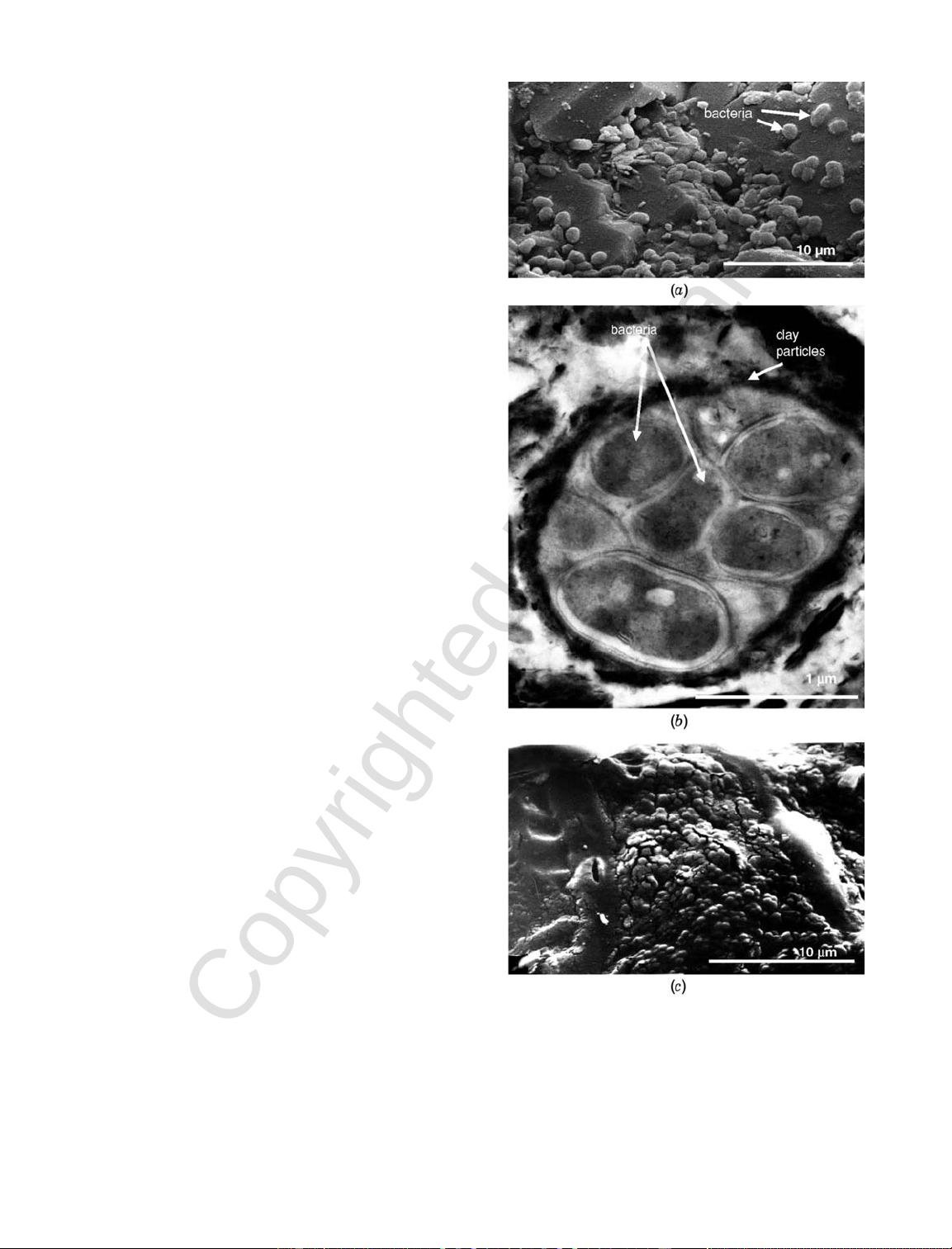
Bacteria tend to adhere to mineral surfaces and form
microcolonies known as biofilms as shown in Fig. 2.7c.
Some biofilms are made of single-type bacteria, while
others involve symbiotic communities where two or
more bacteria types coexist and complement each
other. For example, biofilms involved in rock weath-
ering may involve an upper aerobic layer, followed by
an intermediate facultative layer that rests on top of the
aerobic layer that produces the weathering agents
(e.g., acids) directly on the rock surface (Ehrlich,
1998). Biofilms bind cations in the pore fluid and fa-
cilitate nucleation and crystal growth even at low ionic
concentrations in the pore fluid (Konhauser and Urru-
tia, 1999). After nucleation is initiated, further mineral
growth or precipitation can occur abiotically, including
the precipitation of amorphous iron–aluminum sili-
cates and poorly crystallized claylike minerals, such as
allophone, imogolite, and smectite (Urrutia and Bev-
eridge, 1995; Ehrlich, 1999; Barton et al., 2001).
In the case of the Carsington Dam construction,
Cripps et al. (1993) hypothesized that autotrophic bac-
teria greatly accelerated the oxidation rate of the pyrite,
so that it occurred within months during construction.
The resulting sulfuric acid reacted with the drainage
blanket constructed of carboniferous limestone, which
then resulted in precipitation of gypsum and iron hy-
droxide, clogging of drains and generation of carbon
dioxide.
Weathering Products
The products of weathering, several of which will gen-
erally coexist at one time, include:
1. Unaltered minerals that are either highly resistant
or freshly exposed
2. Newly formed, more stable minerals having the
same structure as the original mineral
3. Newly formed minerals having a form similar to
the original, but a changed internal structure
4. Products of disrupted minerals, either at or trans-
ported from the site. Such minerals might include
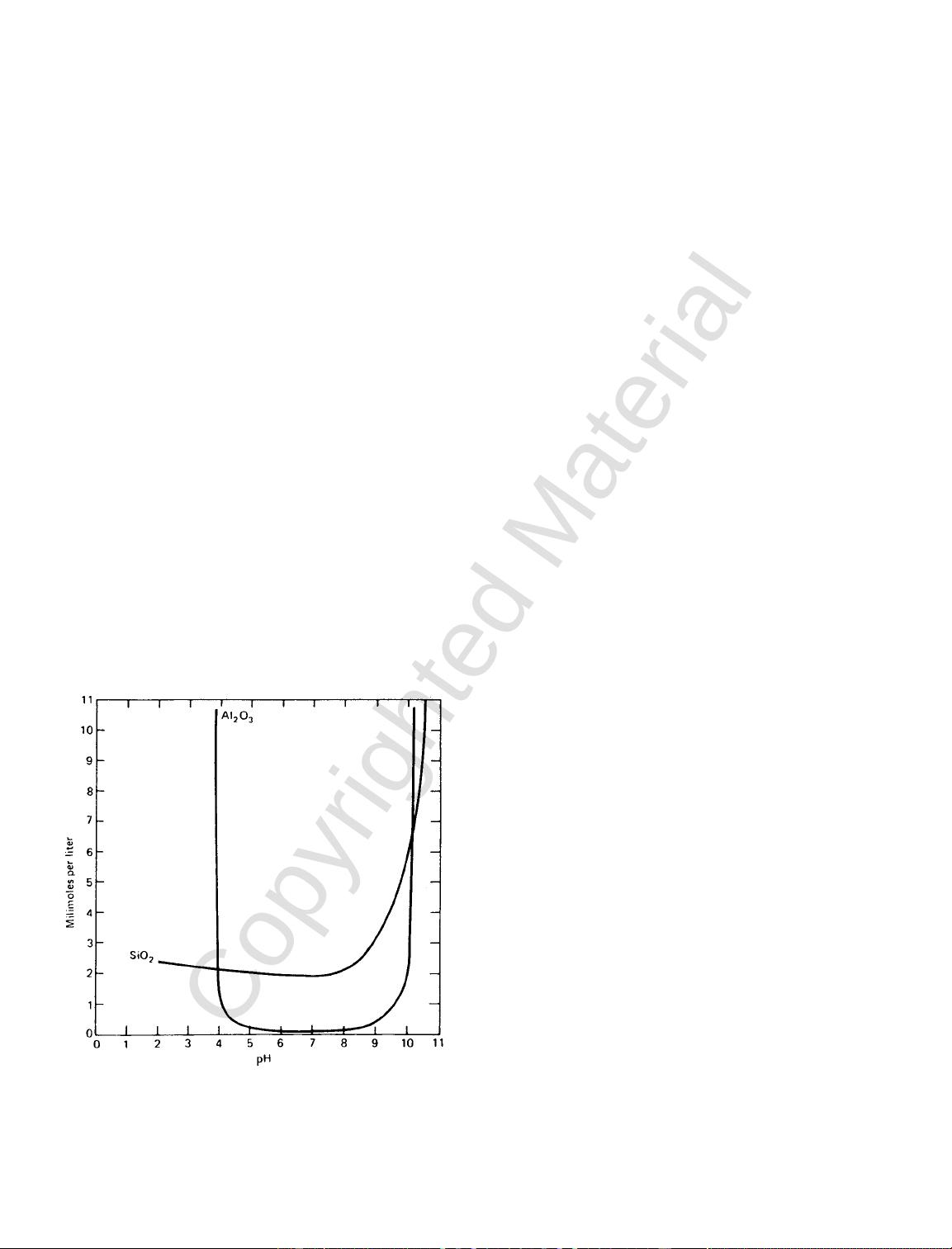
a. Colloidal gels of Al
2
O
3
and SiO
2
b. Clay minerals
c. Zeolites
d. Cations and anions in solution
e. Mineral precipitates
5. Unused guest reactants
The relationship between minerals and different
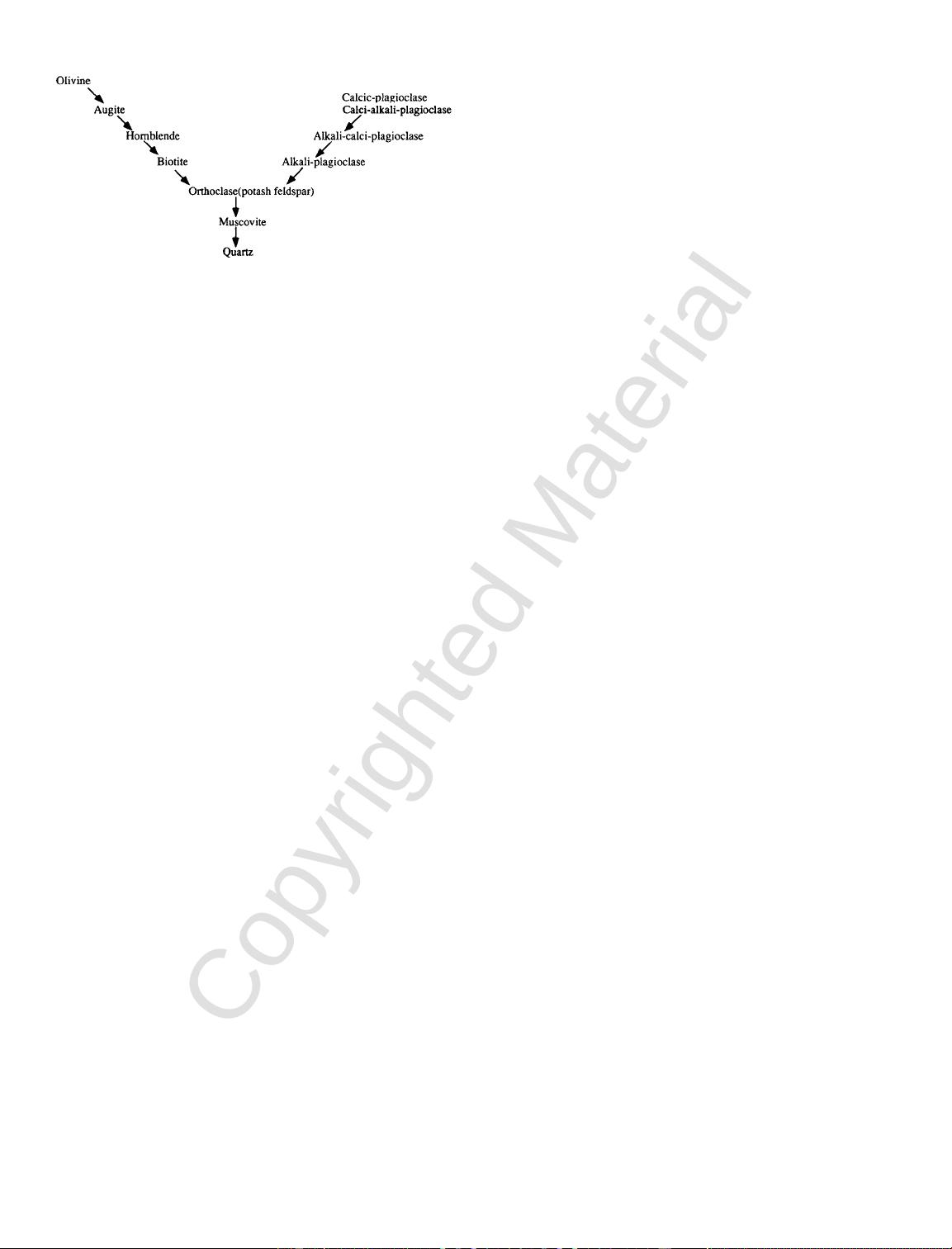
weathering stages is given in Table 2.2. The similarity
between the order of representative minerals for the
different weathering stages and Bowen’s reaction se-
ries given earlier (Fig. 2.5) may be noted.
Contrasts in compositions between terrestrial and lu-
nar soils can be accounted for largely in terms of dif-
ferences in chemical weathering. Soils on Earth are
composed mainly of quartz and clay minerals because
the minerals of lower stability, such as feldspar, oli-
vine, hornblende, and glasses, are rapidly removed by
chemical weathering. On the Moon, however, the ab-
sence of water and free oxygen prevent chemical
weathering. Hence, lunar soils are made up mainly of
fragmented parent rock and rapidly crystallized
Copyrighted Material
Copyright © 2005 John Wiley & Sons Retrieved from: www.knovel.com