VOLUME
9,
NUMBER
2,
APRIL
2003
121
appears to be decreasing with increasing film flow rate, these results seem to corroborate each
other. This may suggest that the results shown by Fujita (1993) were also influenced by incom-
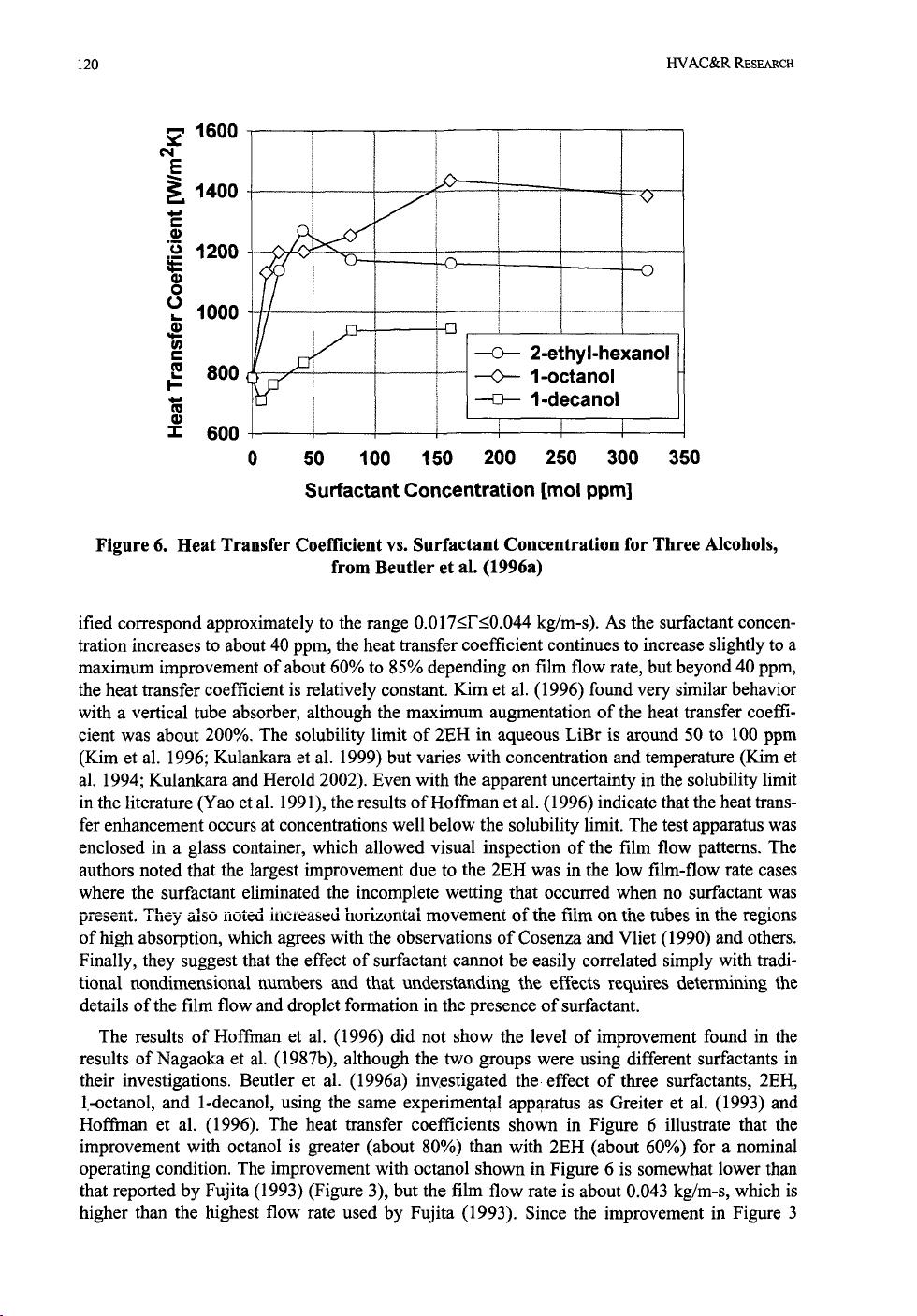
plete surface wetting at the low flow rates without surfactant. Beutler et al. (1996a) note that all
three alcohols studied lower the surface tension of aqueous LiBr and that this reduction
increases steadily up to their respective solubility limits. This trend in surface tension does not,
however, adequately explain the trends shown in Figure 6. Moreover, they point out that the
sur-
face tension is nearly the same with 1-octano1 as with 1-decano1 at high concentrations. There-
fore, they conclude that surface tension reduction is not a sufficient parameter for explaining the
absorption enhancement. Kulankara and Herold (2000) suggest that this might be because the
vapor pressures of 2EH and octano1 are 12 and
7
times higher than 1-decanol, resulting in rela-
tively little 1 -deconal in the vapor to be adsorbed onto the film surface. This phenomenon, there-
fore, provides one possible explanation of the results shown in Figure 6. Unfortunately, Beutler
et al. (1996a) do not estimate the mass transfer coefficients,
so
there is no corroboration of
results shown in Figure 4.
investigators such as Kim et al. (1994) and Yuan and Herold (2001) have pointed out that the
surface tension in the presence of surfactants depends on the age of the surface and the constitu-
ents of the surrounding vapor, and that transient measurements of surface tension yield signifi-
cantly different results than steady-state measurements. The reduction of surface tension due to
surfactants increases with the age of the surface, although the duration of this delay depends on
the surfactant. Moreover, Kulankara and Herold (2000), Yuan and Herold (2001), and Kyung
and Herold (2002) suggest that the arrival of surfactant to the surface from the vapor phase is
more influential than the concentration in the liquid phase as will be discussed below. Also, Zie-
gler et al. (1 999) use the apparatus of Greiter et al. (1 993) to show that the heat transfer coeffi-
cient actually increases as the temperature driving force increases, which suggests a two-way
coupling between the effect of surfactant
on
the absorption rate; that is, the enhancement of
absorption rates due to the surfactant depends on the rate of absorption. Kyung and Herold
(2000) show similar trends using an entire single-effect absorption system. Additionally, Ziegler
et al. (1999) show that almost all of the enhancement associated with 2EH occurs with concen-
trations as low as a fraction of a ppm. As expected, since surfactants primarily influence the
behavior at interfaces, the bulk concentration of surfactant is not as relevant as its concentration
at the film interface in determining the effect of surfactant on absorption (Kulankara et al. 1999).
Atchley et al. (1998) and Miller (1999) both investigate the effect of 2EH on absorption over
six horizontal tubes; these two reports are based on data taken from the same test apparatus.
Although their test setup has a comparatively low coolant-side heat transfer coefficient (similar
to Cosenza and Vliet
[
1990]), their results corroborate some of the findings mentioned above. In
addition, the authors present photographs of the change in flow pattern when
500
ppm 2EH is
added. The improvement in heat transfer coefficient due to the addition of surfactant reported by
the authors is nominally about 200% to 300%, which is higher than reported above. However,
this result is obtained with a lower solution flow rate (0.009 kgím-s) and a higher inlet LiBr con-
centration (60% to 62%) than the previous results. Nagaoka et al. (1987b) showed that the
improvement due to surfactant increases as the film flow rate decreases. This trend can also be
seen indirectly in some of the results of Atchley et al. (1998) and Miller (1999). Kim et al.
(1 994) suggest that for a given concentration of surfactant, the surface tension reduction
increases as the LiBr concentration increases. Thus, it is plausible that the larger improvement
measured by Atchley et al. (1 998) and Miller (1999) is a combined result of the lower solution
flow rate and higher inlet concentration. By varying the coolant inlet temperature and, thus, the
total absorption duty, the authors show that without surfactant, the heat transfer coefficient
remains nearly constant irrespective of absorber load. However, with surfactant, the heat trans-
fer coefficient increases steadily with increasing absorption rate, the same coupling observed by
Ziegler et al. (1 999). The authors also note that the effect of the 2EH on the flow pattern changes
Provided by IHS under license with ASHRAE
Licensee=Naval Aviation Depot/5918326100
Not for Resale, 01/19/2008 02:25:05 MST
No reproduction or networking permitted without license from IHS
--`,`,`,``,,`,`,,,`,``,,,`,`,,``-`-`,,`,,`,`,,`---