and broad emission bands. And fluorescent dyes used as indicators in
paper-based devices are required to change colors or be quenched after
adding analytes, and especially the complexity of the synthesis and
purification of organic molecules.
2.2. Quantum dots
Quantum dots (QDs) are highly fluorescent inorganic semi-
conductor nanocrystals in zero dimension with a diameter ranging from
2to10nm(Fig. 3a) (Chan and Nie, 1998). The QDs consist of hundreds
to thousands of atoms from group II-VI (e.g., CdSe and CdTe), group III-
V (e.g., InP and InAs), or group IV-VI (e.g., PbSe). The surface-naked
QDs usually show low fluorescence quantum yield, mainly induced by
the presence of surface defects. To address this issue, QDs with the core-
shell configuration (three types as shown in Fig. 3b) and molecules
coating become attractive in deactivating the surface trap state. QDs of
type 1 with a higher band gap, energy gap between the conduction
band (CB) and valence band (VB), as the shell and lower band gap as
the core tend to concentrate carriers within the core. Type 2 is just the
opposite. Type 3 means the CB or VB of the core within the band gap of
the shell (Stanisavljevic et al., 2015). Different from fluorophores, an
electron of QDs will be excited from the VB to the CB generating a hole
in VB and forming an electron-hole pair after absorption of a photon.
The recombination of electron-hole pairs will produce fluorescence.
The QDs have broad excitation band, symmetric emission spectra,
narrow defined tunable emission peak, long fluorescence lifetime, and
large Stokes' shifts (Zhou et al., 2005). Various colors are available by
changing the size, shape, and composition of the QDs. These char-
acteristics allow QDs to be a great alternative for paper-based diag-
nostics to detect ions (Wang et al., 2015b), nucleic acids (Duran et al.,
2016), proteins (Li et al., 2016), cells (Liang et al., 2016a), etc.
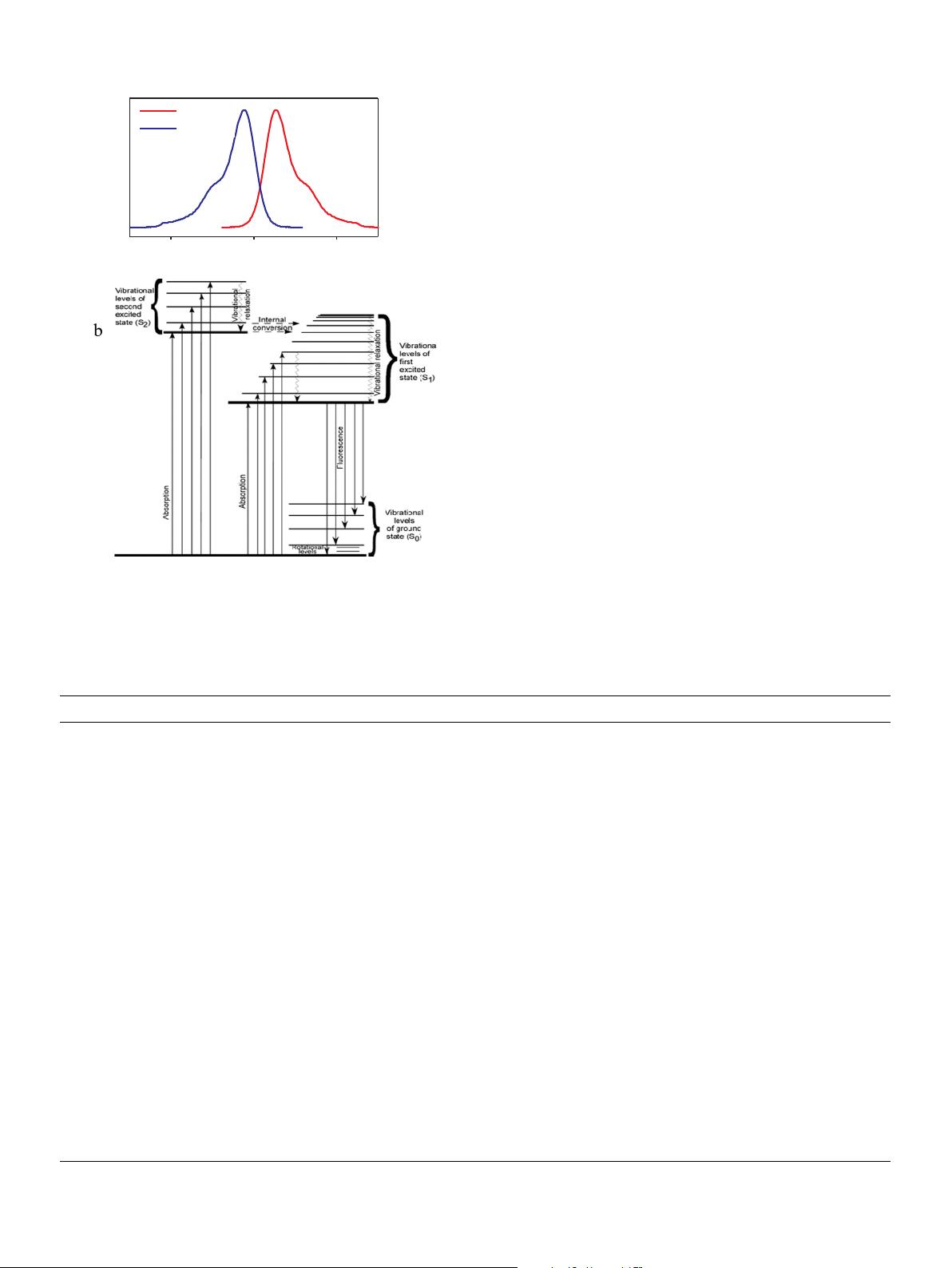
a
400 500 600
Wavelength(nm)
Emission
Absorption
Fig. 1. (a) Absorption and emission spectra of Rhodamine showing the Stokes' shift. (b)
Jablonski diagram showing the energy level of a generic molecule and the generation of
fluorescence (Noomnarm and Clegg, 2009).
Table 1
Comparison of different fluorescent materials used in the paper-based fluorogenic devices.
Fluorescent materials Advantages Disadvantages Applications
Fluorescent dyes a) Small size
b) Easy surface modifications
c) Good biocompatibility
a) Low fluorescence quantum yield
b) Instable fluorescence and rapid
photobleaching
c) Autofluorescence
d) Light scattering
Neisseria meningitides (Dou et al., 2014), Single-stranded DNA
(Scida et al., 2013), β-D-galactosidase (Thom et al., 2014),
Adrafinil (Caglayan et al., 2016), Alkaline phosphatase (Koo et al.,
2014), Polyols (Ikeda et al., 2012), Al
3+
(Kim et al., 2015), Heavy
metals (Feng et al., 2013), Nitroaromatic explosive (Taudte et al.,
2013), Ammonia gas (Lin et al., 2015), Hypochlorite (Lee et al.,
2015)
Quantum dots a) High quantum yield
b) Stable fluorescence
c) High Stoke shift
d) Tunable emission wavelength and
narrow emission spectra
a) Toxicity
b) Optical blinking
c) Light scattering
d) Low light penetration
Staphylococcus aureus (Chandan et al., 2016), Nucleic acid
hybridization (Noor and Krull, 2014), Nitrated ceruloplasmin (Li
et al., 2010), Cysteine and homocysteine (Wang et al., 2015a),
Tyrosinase (Yan et al., 2015), Peptides (Petryayeva and Algar,
2013), Liver cancer cell (Wu et al., 2015), H
2
O
2
(Duran et al.,
2016), Cu
2+
(Wang et al., 2015b), Nitroaromatic explosive (Zhang
et al., 2011), Methyl viologen (Su et al., 2015b)
Metal nanoclusters a) Tunable and strong fluorescence
emission
b) Small size
c) Non-toxicity and biocompatibility
a) Difficulties to obtain
monodispersed metal clusters
Rifampicin (Chatterjee et al., 2015), Organophosphate (Zhang
et al., 2014), Cu
2+
(Liu et al., 2012), Hg
2+
(Qi et al., 2015),
Nitroaromatic explosive (Ma et al., 2012)
Upconversion
nanoparticals
a) High photostability and quantum
yield
b) Zero auto-fluorescence background
for the improved signal-to-noise ratio
c) Large anti-stokes shifts
d) Multicolor-emission capability
e) Biocompatibility
a) Near-infrared light with high
density as the excitation resource
Nucleic acid hybridization (Ju et al., 2014; Noor and Krull, 2013),
Multiple cancer (Xu et al., 2016)
Carbon dots a) Strong fluorescence
b) Low toxicity and good
biocompatibility
c) Good water solubility
d) Tunable emission
e) Easy functionalization
a) The uncertain origins of
fluorescent emission
Copper ions (Wang et al., 2016); Pb
2+
(Abhishek et al., 2016);
Al
3+
(Kim et al., 2015); Hg
2+
( Zou et al., 2016; Yuan et al., 2013);
DNA (Wang et al., 2013); HIV (Kurdekar et al., 2016)
M. Wu et al.
Biosensors and Bioelectronics 102 (2018) 256–266
258









