Development of a Novel Markov Chain Model for
Oxidative-dependent CaMKIIδ Activation
Shanzhuo Zhang
1
, Qince Li
1
, Lufang Zhou
2
, Kuanquan Wang
1
, Henggui Zhang
1,3
1
Harbin Institute of Technology, Harbin, China
2
University of Alabama, Tuscaloosa, USA
3
University of Manchester, Manchester, UK
Abstract
Dysfunction in the Calcium (Ca
2+
)-calmodulin (CaM)
dependent kinase II (CaMKII) signalling can lead to
several pathologies, such as heart failure and arrhythmia.
Especially, the role of CaMKII signalling in oxidative
stress-induced arrhythmias remains unclear. In this
study, we aimed to develop a new Markov chain model of
CaMKII δ-isoform (CaMKIIδ) that involves both of the
autophosphorylation and oxidation pathways to better
simulate CaMKII signalling under oxidative stress in
cardiomyocytes. Based on the four-state model developed
by Chiba et al., we implemented two oxidized states
including a Ca
2+
/CaM-bound state and a Ca
2+
/CaM-
dissociated state, representing the new pathway of
oxidation-dependent activation. Using the model, we
reproduced the CaM affinity to CaMKIIδ, the dependence
of autophosphorylation on CaM. The frequency-
dependent activation of CaMKII was simulated for both
CaMKII α- and δ-isoforms. For the oxidation pathway,
our simulation suggested that H
2
O
2
increased the kinase
activity in a dose-dependent manner, which also fitted to
experimental data. Finally this model was incorporated
in a human atrial cell model to simulate the effects of
CaMKII activation on cellular action potentials.
1. Introduction
Ca
2+
/CaM dependent protein kinase II (CaMKII) plays
a key role in connecting upstream cellular signals to
cellular behaviours. Dysfunction of CaMKII under
reactive oxygen species (ROS) has been found in many
pathologies, including heart failure, apoptosis, hyper-
trophy, and myocardium infarction. Recently, new
findings [1] on the oxidative activation pathway of
CaMKII further expands our horizons in understanding
the mechanism responsible for dysfunctional CaMKII-
induced heart diseases.
It has been shown that oxidized CaMKII can trigger
atrial fibrillation [2]. However, to our best knowledge, it
is incompletely understood yet the role of oxidative
CaMKII in the genesis of atrial arrhythmias.
In this study, we (1) developed a novel CaMKII
Markov chain model that considered the oxidative
activation pathway; (2) fitted parameters of this model to
better reproduce the experimental data compared with
other studies; (3) incorporated this CaMKII model into a
human atrial cell model to investigate its impact on
human atrial action potential.
2. Methods
2.1. Model structure
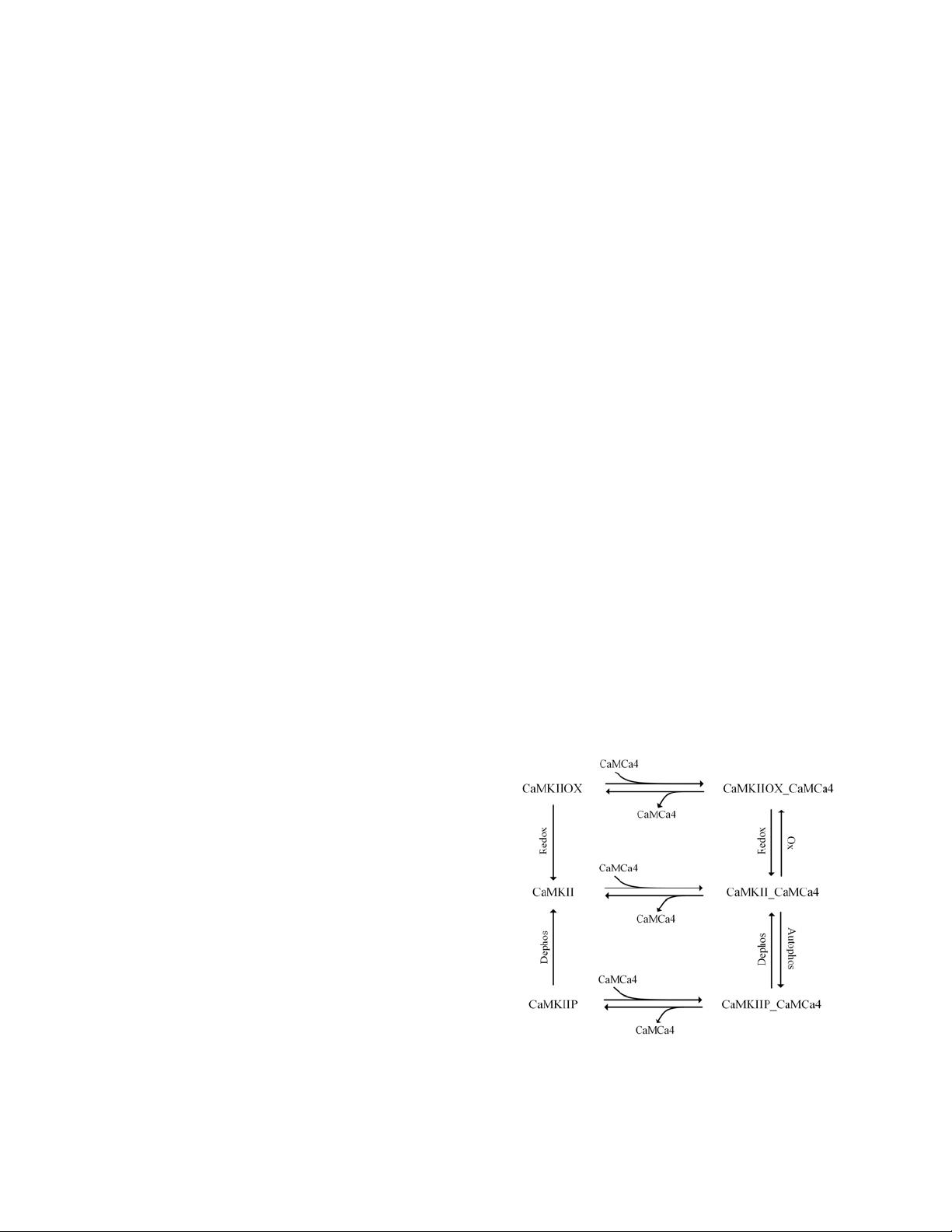
A CaMKII holoenzyme is usually assembled by 8-14
CaMKII subunits. A single CaMKII has 3 domains
named the association domain, the regulatory domain,
and the catalytic domain. Binding of a CaMCa4 (a CaM
binding with 4 Ca
2+
) to the regulatory domain exposes the
catalytic domain, which means its activation (state
CaMKII_CaMCa4 in Figure 1). Under this state and in
the presence of ATP, CaMKII can be further
autophosphorylated, and then gets long lasting activity
even upon dissociation of CaMCa4. In addition, recent
study [1] has pointed out that CaMKII under the
Figure 1. A schematic illustration of our CaMKII model.
There is only one inactive state (CaMKII) in this model,
other states including three Ca
2+
/CaM bounding states
and two phosphorylated or oxidative states are all active.
Computing in Cardiology 2015; 42:881-884.









