regarding axon guidance, axon sorting and axon position-
ing (Sakano, 2010; Zou et al., 2009).
In different species, each glomerular structure results
from the convergence of 5 to 40 thousand axon terminals
of sensory neurons that express the same odorant recep-
tor (Figure 1.2). Therefore, the glomerular layer can
be considered as a two-dimensional anat omical repre-
sentation of the olfactory receptors’ repertoire (also
called ‘chemotopic map’; Johnson and Leon, 2007;
Wachowiak and Shipley, 2006). Because one odor can ac-
tivate several olfactory neurons, odor information is
first encoded by the combinatorial patterns of glomeru-
lar activation. Odorants activate a specific array of olfac-
tory sensory neurons that lead to a chemotopically
fragmented map of activated glomeruli on the surface
of the olfactory bulb (Me ister and Bonhoeffer, 2001).
Remarkably, the precise projection pattern can be repro-
duced from one animal to another and even between
different rodent species (Soucy et al., 2009). Distinct
odorants activate different combinations of glomeruli
and two odors are more difficult to discriminate when
these show a greater overlap in this glomerular chemo-
topic map (Linster et al., 2001). Nevertheless, if such a
spatial pattern coding scheme were only applicable to
several odors, it would not be able to provide a suffi-
ciently large coding space to discern between the mil-
lions of potential odors or mixtures of odor present in
our environment (Laurent, 2002).
The sensory neurons project to paired olfactory glo-
meruli on both the medial and lateral aspects of the olfac-
tory bulb, thus creating two mirror-symmetric maps
(Mombaerts et al., 1996). As each group of glomerulus-
specific output neurons is odorant receptor-specific,
glomeruli form a morphological as well as functionally
defined network somewhat analogous to barrels in the
somatosensory cortex (Johnson and Leon, 2007). In
mammals, the convergence ratio of sensory neurons
to olfactory bulb output neuron is very large: about
1000:1 (Zou et al., 2009). A bulbar output neuron thus
forms its responses to odors from very large numbers
of converging inputs, ensuring detection of faint signals,
increase signal-to-noise ratios and temporal noise
average.
1.2.2 Synaptic Processing Within Olfactory Bulb
Microcircuits
Because of its relatively simple anatomical organiza-
tion and easy access, the olfactory bulb has been a privi-
leged model system for deciphering the principles
underlying ne twork processing of sensory information.
There, odors elicit a well-organized pattern of glomeruli
activation across the surface of the olfactory bulb, but the
mechanisms by which this chemotopic map is processed
into an odor code by the bulbar circuitry has recently
attracted more attention. With the advance in recent
years of in vitro brain slice preparation, as well as in vivo
recording techniques that were applied on behaving an-
imals, the complex processing of the olfactory informa-
tion is starting to be revealed. Since Cajal’s pioneering
studies, it has been known that the main output neurons
of the bulb, the so-called mitral cells, are located in a sin-
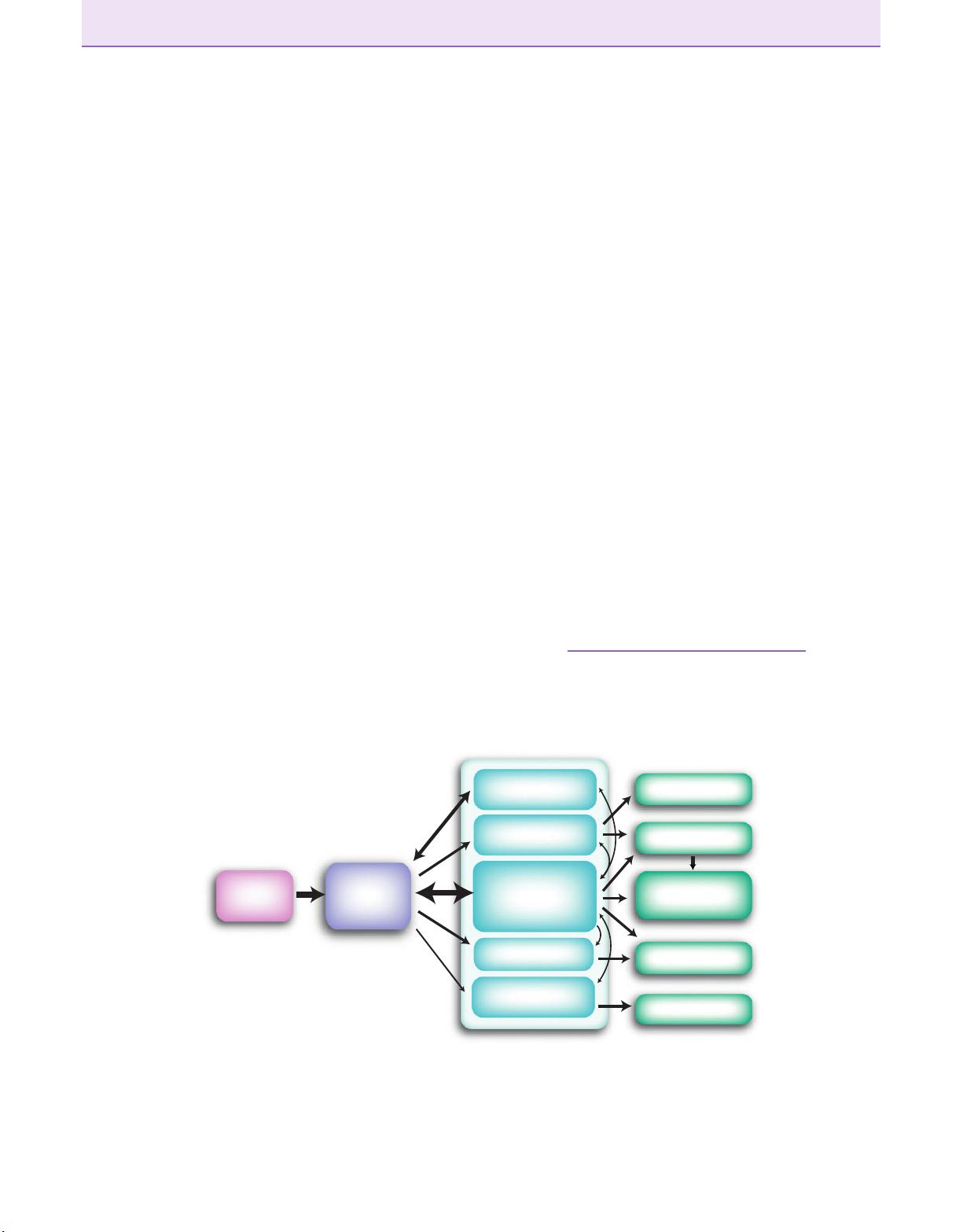
gle lamina, the mitral cell layer (Figure 1.3). A second
population of output neurons, namely tufted cells, are
scattered in the external plexiform layer (EPL). The pri-
mary (or apical) dendrite of mitral and tufted cells,
extending vertically from its soma, contacts one glomer-
ulus, whereas their multiple long secondary dendrites
spread in the EPL.
About 50–100 output neurons (mitral/tufted cells)
emanate from each glomerulus and project to a number
of higher centers that compose the olfactory cortex (see
Figure 1.1). Output neurons are the backbone of two se-
rial intrabulbar microcircuits: one between primary api-
cal dendrites and juxtaglomerular cells and the other
between secondary dendrites and granule cells. The
main difference between juxtaglomerular and granule
cells is that the former mediate mostly interactions be-
tween cells affiliated with the same glomerulus, wh ile
granule cells mostly mediate interactions between out-
put neurons projecting to many different glomeruli
(Figure 1.3). Therefore, two potential distinct sites of
odor processing can be distinguished according to the to-
pographical organization of the bulbar circuit. The first
one resides in the glomeruli where local interneurons
shape excitatory inputs coming from sensory neurons.
The second one lies in the EPL, where reciprocal dendro-
dendritic synapses are heavily distrib uted between den-
dritic spines of local interneurons and the dendrites of
output neurons. These two inhibitory microcircuits are
also under the control of centrifugal fibers coming from
cortical and neuromodulatory area.
1.2.2.1 Synaptic Transmission at the First Synapses
The glomerular layer constitutes a first site of integra-
tion for olfactory information. In this layer, axonal ter-
mini of olfactory sensory neurons synapse directly
onto output neurons (50–100 cells per glomerulus)
and also onto local neurons, namely juxtaglomerular
cells (1000–2000 cells per glomerulus). The olfactory
nerve-evoked excitatory responses of both neuron types
comprise fast amino-3-hydroxy-5-methyl-4-isoaxozole-
propionic acid (AMPA) and slow N-methyl-
D-aspartate
(NMDA) components. The latter is particularly long-
lasting and may play an important role in the bulbar out-
put by maintaining a pattern of sustained discharge of
output neurons (Carlson et al., 2000).
6 1. THE FORM AND FUNCTIONS OF NEURAL CIRCUITS IN THE OLFACTORY BULB
I. CIRCUIT DEVELOPMENT