ZnO-NANOWIRES COATED WITH GAMMA-ORDERED MESOPOROUS
ALUMINA (GAMMA-OMA) CATALYST FOR DETECTION OF FREON
Pengcheng Xu, Ying Chen, and Xinxin Li
State Key Lab of Transducer Technology, Shanghai Institute of Microsystem and Information
Technology, Chinese Academy of Sciences, 865 Changning Road, Shanghai 200050, CHINA
ABSTRACT
In this work, gamma-phase ordered mesoporous
alumina (J-OMA) is used as key catalyst to realize the
sensitive detection of Freon R134a with ZnO micro-sensor.
Due to the inert characteristic, Freon R134a (CH
2
FCF
3
) is
difficult to be detected by using conventional oxide
semiconductor gas sensors. J-OMA catalyst exhibits
satisfied active to R134a and produces several kinds of
active radicals. The ZnO nanowire (ZnO NW) sensor is
sensitive to the highly active radicals. The kinds of radicals
are qualitative identified by mass spectrum (MS) and the
J-OMA catalyst-enhanced sensing mechanism is clearly
elucidated.
INTRODUCTION
In the past decades, metal oxide semiconductors such
as tin dioxide (SnO
2
), zinc oxide (ZnO) and indium oxide
(In
2
O
3
) are widely used as sensing materials for
gases/vapors detection [1-4]. By using metal oxide sensing
materials, various kinds of gases/vapors including alcohol,
ketone, carbon monoxide (CO), hydrogen sulfide (H
2
S),
ammonia, and nitrogen dioxide (NO
2
) can be detected. The
universally accepted sensing mechanism between metal
oxide sensing material and target molecules is based on
gas-solid reaction, i.e. the target molecules react with the
adsorbing oxygen species (such as O
2
-
) at the surface of
metal oxide and thereafter, release/capture electrons
to/from the metal oxide sensing material [5]. The electron
releasing/capturing process brings a detectable sensing
signal of resistance change ('R%DVHGRQWKH³gas-solid
reaction´VHQVLQJPHFKDQLVPLWLVGLIILFXOWIRUPHWDOR[LGH
sensors detecting some inert molecules like Freon because
the inert molecule of Freon hardly reacts with the
adsorbing oxygen species without any catalysts.
As a kind of halogenated hydrocarbons, Freon is
widely used as refrigerant in many fields such as domestic
refrigeration and air conditioning. Recognized that the
chlorine contained Freon (i.e. chlorofluorocarbons) has the
negative environmental impacts such as destruction of
stratospheric ozone, Freon of chlorofluorocarbons is
banned in many countries to date. Currently, a new
generation of Freon (hydrochloroflurocarbons, HCFCs)
which features environment friendly characteristic has
been developed. Although it has almost none damage
effect to ozone layer, Freon HCFCs can cause global
warming. In addition, the leakage of Freon HCFCs
decreases the cooling effect of refrigerator which may
cause other problems. For example, one of the typical
Freon HCFCs of R134a (CH
2
FCF
3
) is widely applied in
cold chain industry. In this application field, avoiding
Freon R134a leakage in vehicle is helpful to guarantee the
freshness of the food during the long distance
transportation process. Hence, it is meaningful to real-time
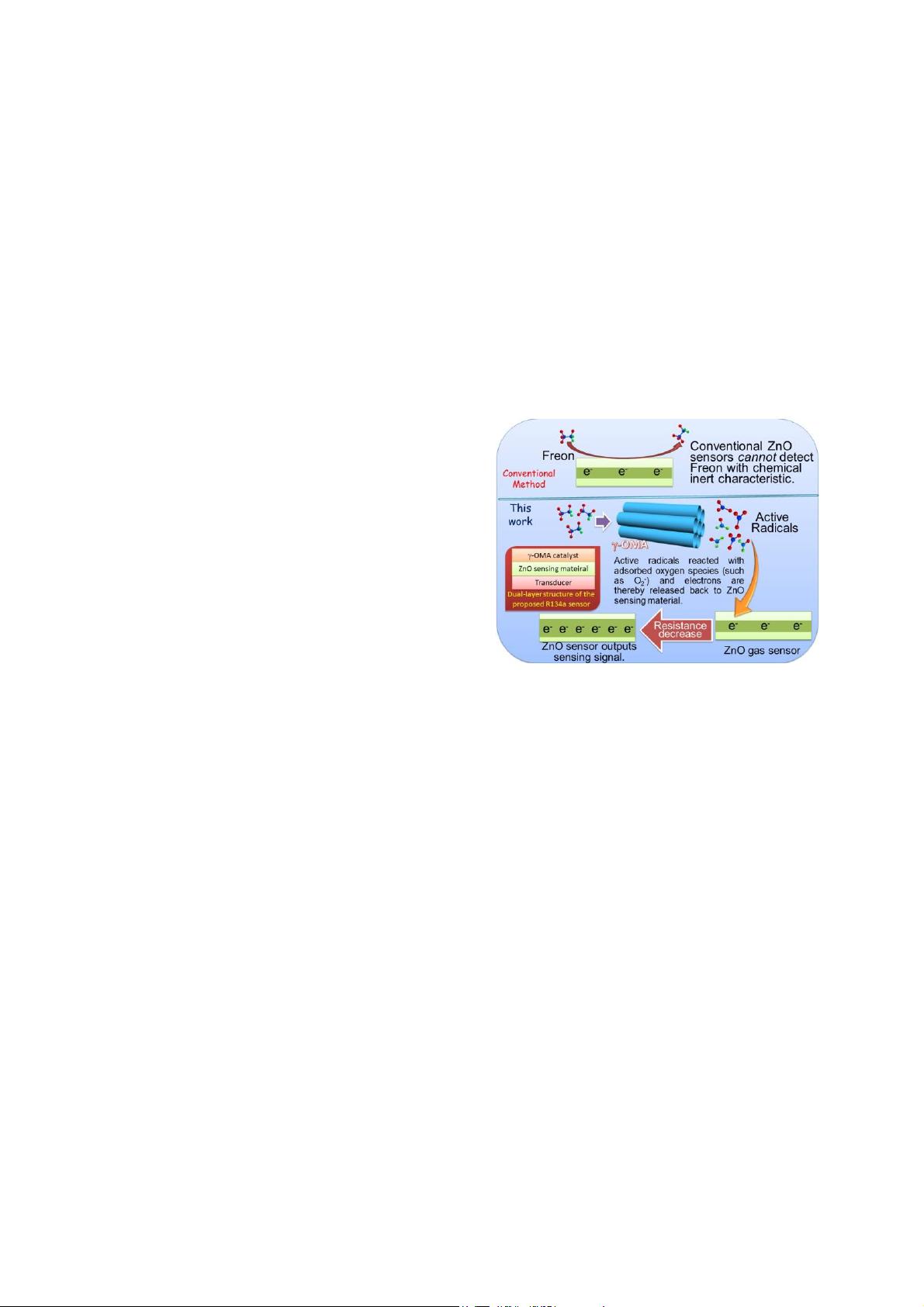
detect low concentration Freon R134a. As is illustrated in
Fig.1, fluorine-contained compound of R134a (CH
2
FCF
3
)
is chemically inert and it cannot directly react with the
adsorbing oxygen species at the surface of metal oxide
sensing material. Thus, it is difficult to detect the leakage
of Freon R134a by using the conventional oxide
semiconductor gas sensors. Developing new catalyst plays
as a key role during the design process of R134a sensors.
Figure 1: Schematically illustration shows the working
principle of the proposed sensor to inert gas of Freon.
Compared with the conventional ZnO sensor,
gamma-phase ordered mesoporous alumina (
J
-OMA)
catalyst is firstly employed to transform Freon into
radicals. Then, the active radicals can be easily detected
by ZnO sensor.
In this study, we find that gamma-phase ordered
mesoporous alumina (J-OMA) exhibits good catalytic
performance to decompose inert Freon R134a molecules.
With the help of J-OMA catalyst, a dual-layer
chemiresistor sensor is designed to detect Freon R134a: i)
nonconductive J-OMA is employed to decompose R134a
to some detectable substances and, ii) ZnO nanowires
(NWs) are used as semiconductor-type sensing material to
detect the produced species. Thus, the sensing response of
ZnO sensor is mainly determined by the produced
intermediate substances. To optimize the sensing
material/catalyst and to elucidate the sensing mechanism,
qualitative identification of the intermediates becomes the
key technology. Herein, on-line mass spectrum (on-line
MS) is used to identified the produced substances in the
exhaust gas. Some sensing products, which cannot be
analyzed on-line, are introduced to special adsorbent and
further off-line identified by GC-MS (Gas
Chromatography-Mass Spectrometer). Fig. 1
schematically shows the advantage of our dual-layer
chemiresistor sensor over the conventional method.
978-1-5386-4782-0/18/$31.00 ©2018 IEEE 920 MEMS 2018, Belfast, Northern Ireland, UK, 21-25 January 2018









