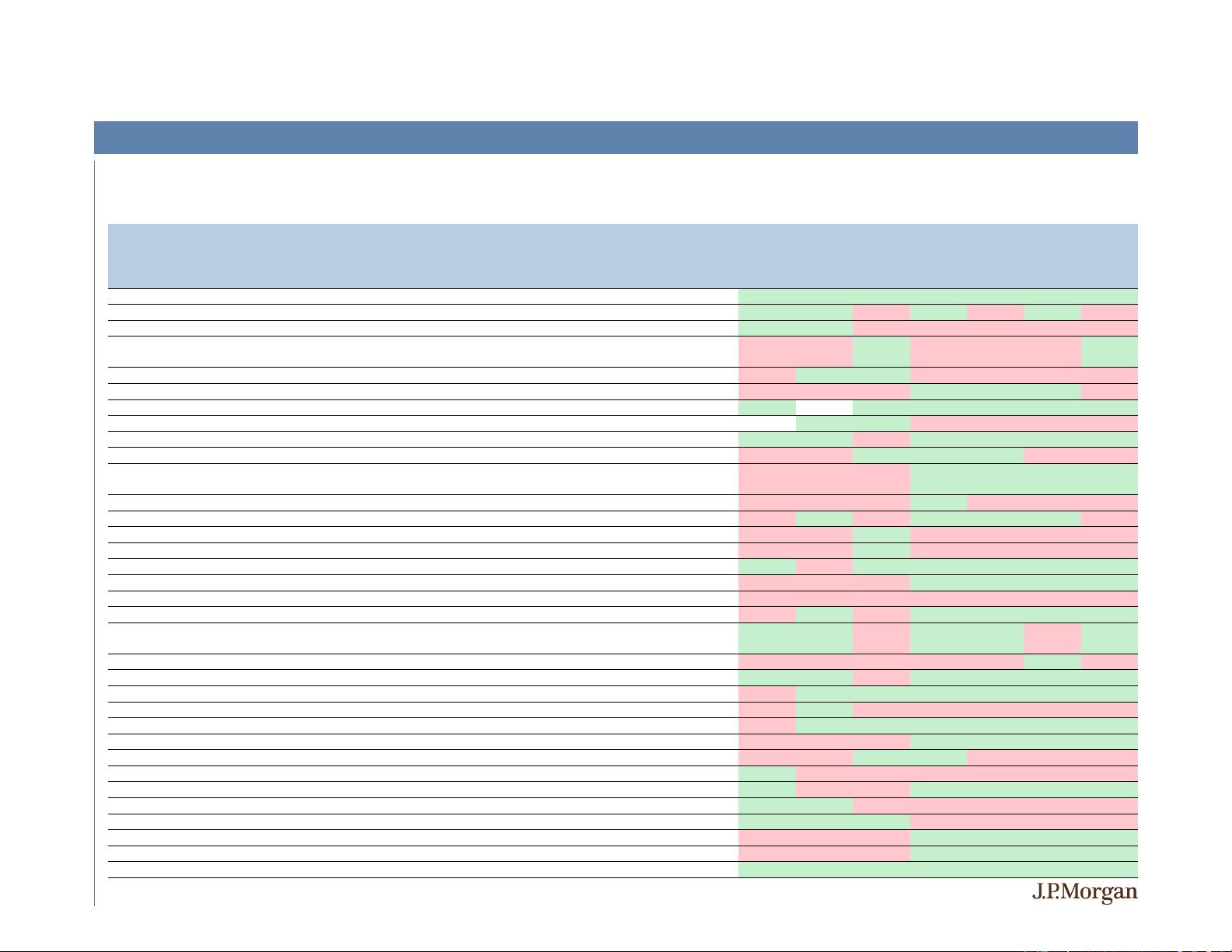
19
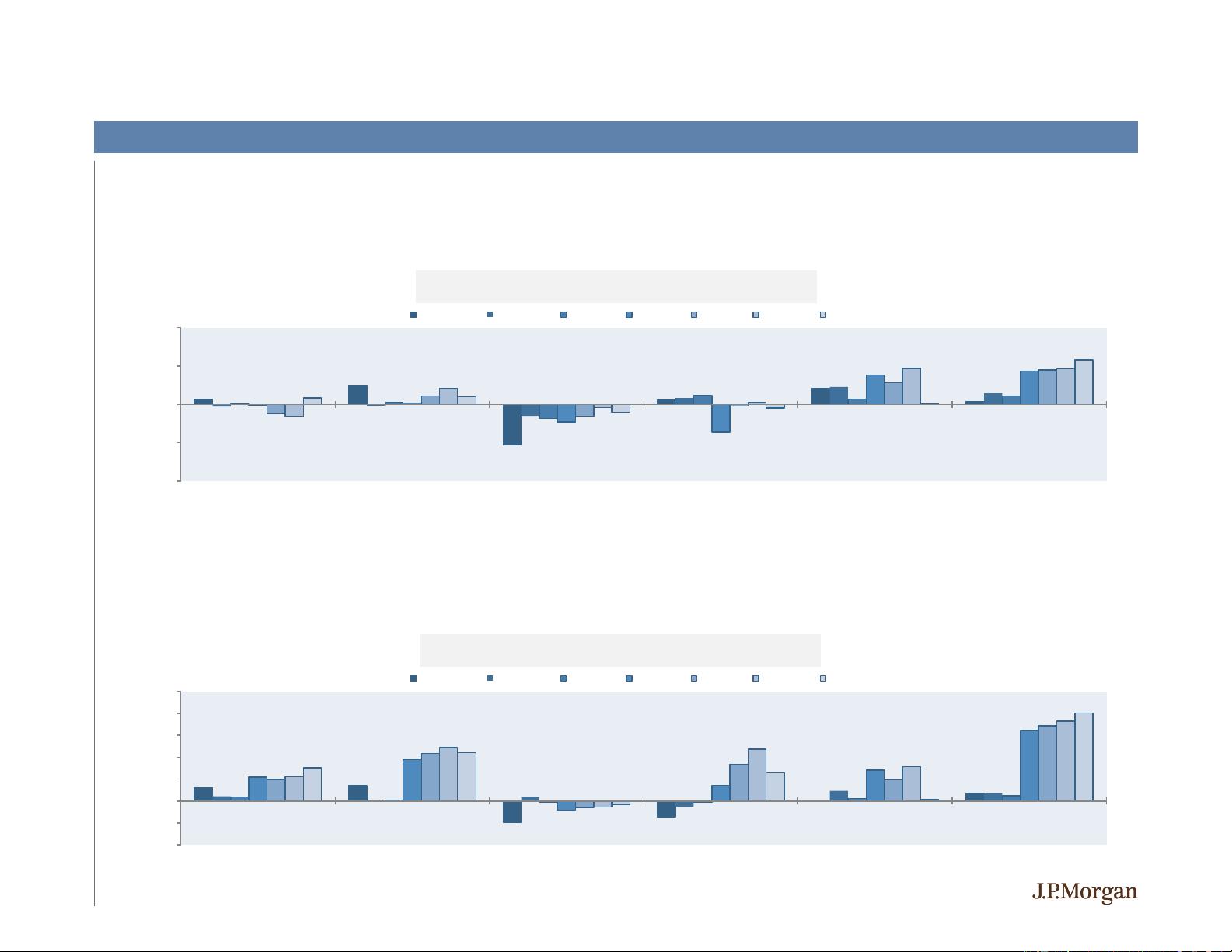
Upside after positive events was higher in 2H19 vs. 1H19 while downside after negative events lessened, potentially reflecting the increased
appetite for playing binary events
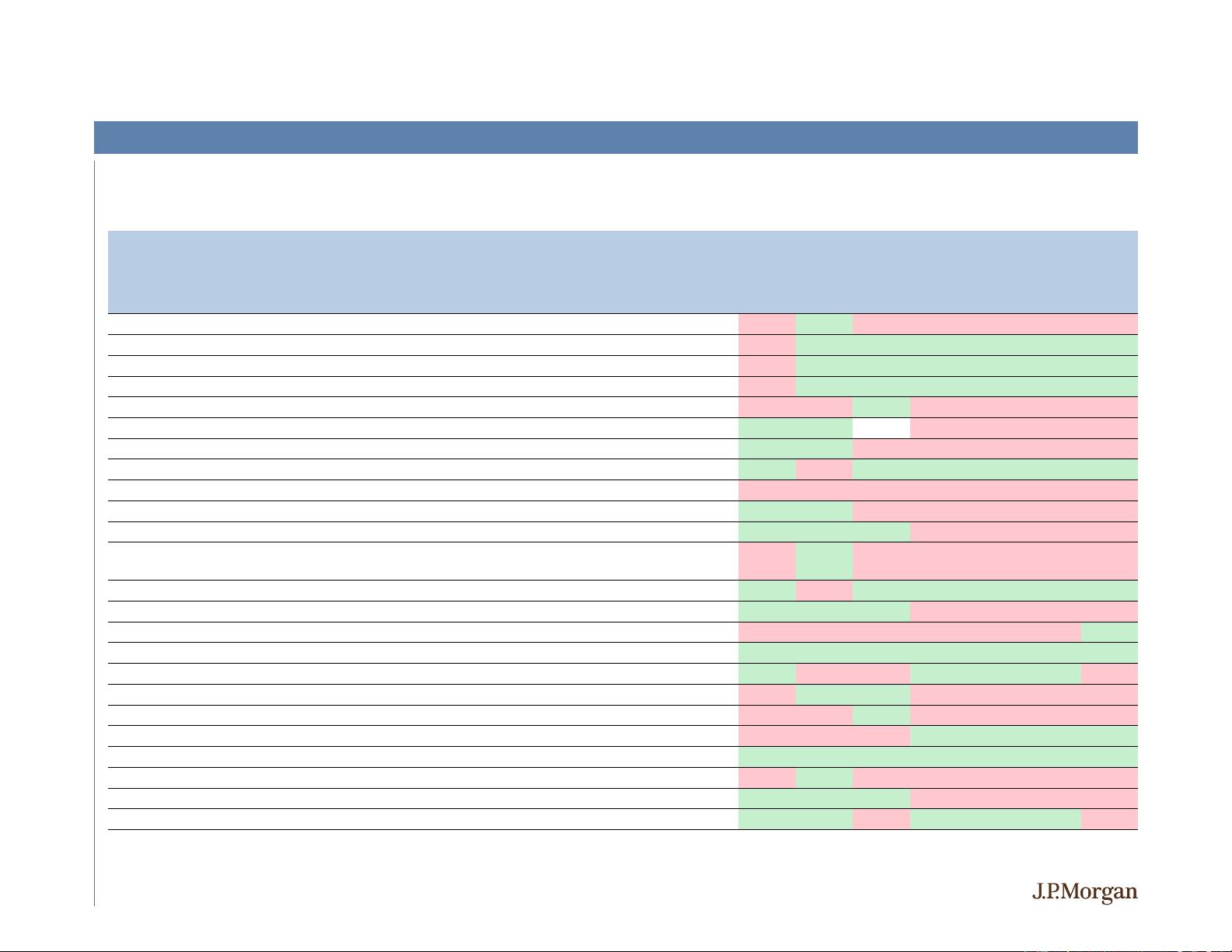
A Quick Look Back at 2019 – Select 2H19 Binary Events
On average, upside after positive events was higher in 2H19 vs. 1H19 whereas downside after negative events was lower
Source: J.P. Morgan Research; Bloomberg as of 12/10/2019.
% Return
-1 Day Day 0 1 Day 5 Days
Xpovio (selinexor) approval for triple
16% 11% 16% 36% 35% 22% 27%
Phase 1/2 ALTA update at ISTH
Topline lumateperone ph III bipolar depression data
11% 4% -1% -13% -15% -17% -34%
Topline data from Phase 3 ENHANCE
-1 study with Nuplazid in schizophrenia
0% -3% 1% -14% -11% -7% 12%
Ad com cancellation for lumateperone in schizophrenia
-10% 5% 1% -32% -32% -32% -28%
Announced renegotiated strategic collab with CELG
-14% -5% -2% 11% 12% 19% -11%
Pegzilarginase Receives BTD in Arginase 1 Deficiency
Sanofi ends collaboration agreement
0% 8% 1% -70% -71% -79% -77%
-MS-2 safety/tolerability study with Vumerity Clinical +
Updates from Phase 1 AAT study and announcement of plans to move VX
CLN6 Gene Therapy Initial Phase 1/2 Update in Batten's Disease
-1% -2% -4% 0% -6% -5% -20%
-12% 0% -2% 14% 10% 27% -9%
Bempeg manufacturing issues / narrower BMS collab
-13% -5% 3% -29% -37% -38% -42%
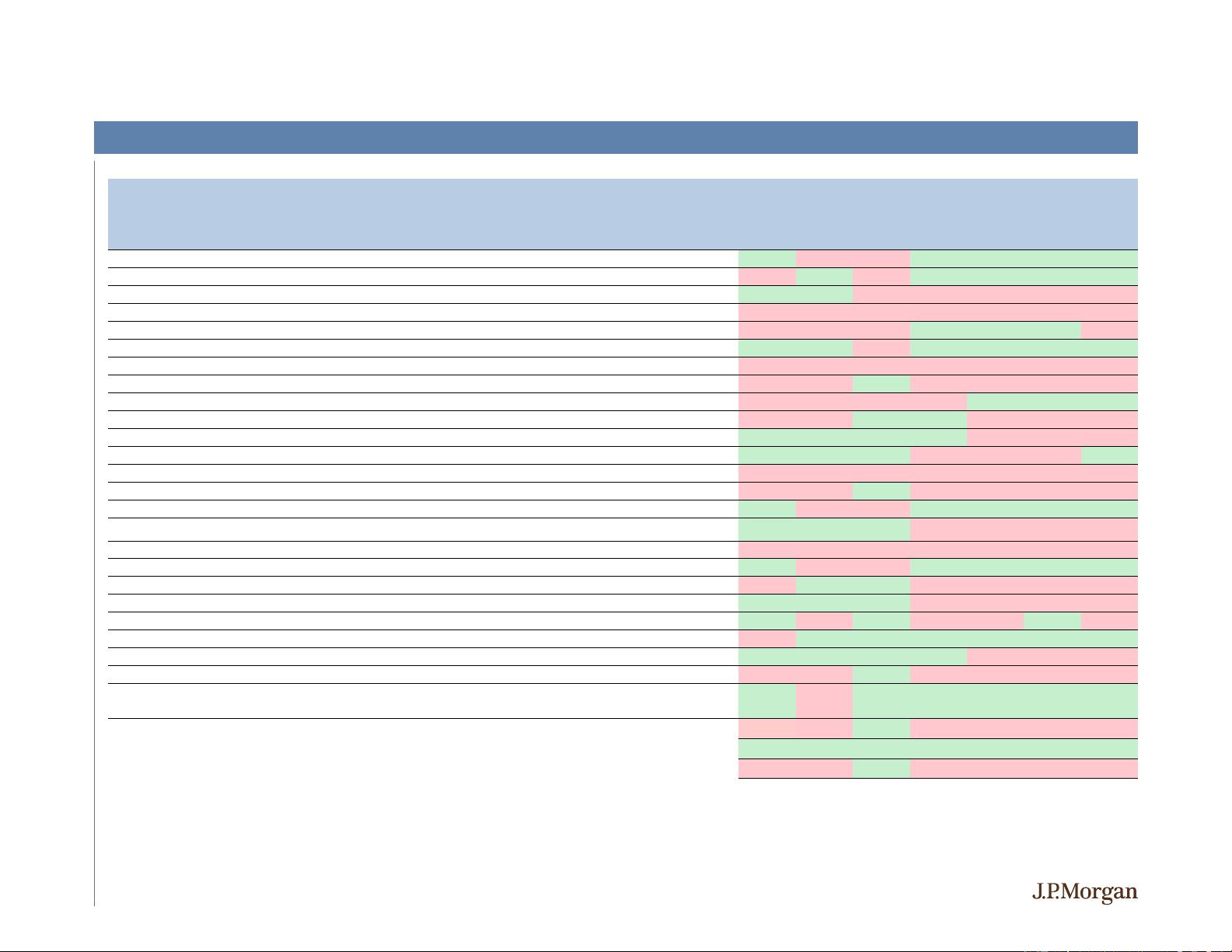
Updated Phase 1 AMG 510 at World Lung (read
-9% -8% 1% -2% -6% -7% -10%
Enbrel litigation with NVS
Topline ripretinib phase III INVICTUS results
-8% -13% -15% 80% 90% 84% 83%
CRL Announced for Golodirsen in exon 53 DMD
-32% -19% -15% -1% -7% -10% -19%
-4% 7% -2% 14% 18% 20% 36%
Inclisiran Phase 3 Topline ORION
-11 Data in ASCVD + elevated LDL-C (read-
IPR institution decision (IPR filed by AMGN)
-12% -13% -10% -6% -3% 3% -3%
-11 presentation at ESC Clinical +
15% 20% -2% 10% 13% 13% 19%
-2% 7% 3% 28% 29% 29% 21%
Data from DTX401 Phase 1/2 second dose cohort
-8% 1% -6% -12% -9% -16% -15%
NDA filing acceptance for accelerated approval (hemolytic anemia endpoints)
Interim data from Phase 3 HARMONY study
-19% -14% -3% 73% 74% 70% 64%
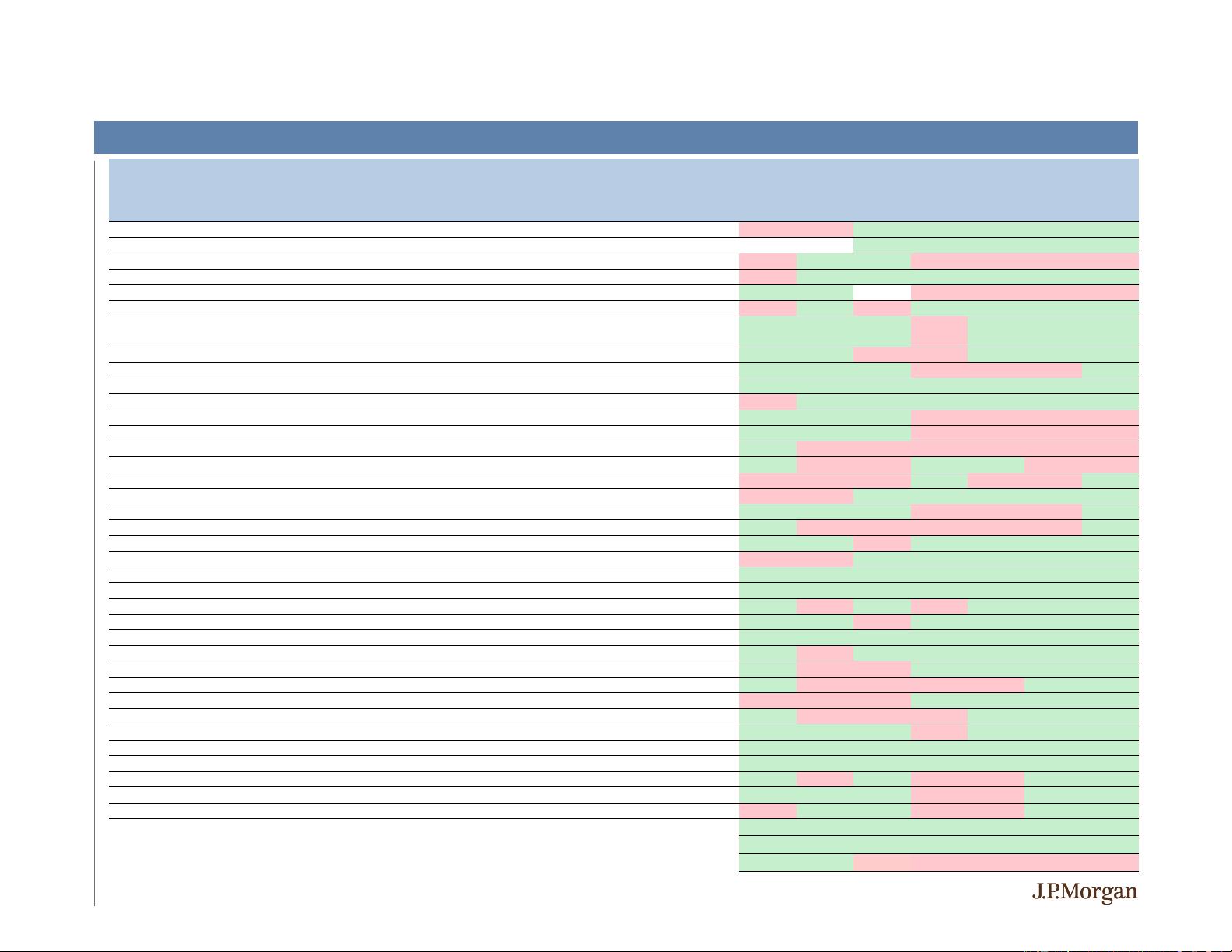
-083 in FSHD phase 2 topline data Clinical -
-3% -2% 2% 0% -3% -4% -7%
-102 for LG UTUC interim analysis Clinical -
5% -10% -1% -16% -23% -28% -34%
-9 and ORION-10 results Clinical +
-1 phase 2a data (EDP-305 for NASH) Clinical -
2% 1% -1% -15% -15% -11% -16%
2% 1% 1% -33% -32% -31% -36%
Multiple Pipeline / Commercial Updates at Analyst Day
-21% -9% -3% 2% 5% 9% 20%
-2% -2% -2% 15% 18% 21% 12%
Topline data in Ph2 study with
tucatinib in metastatic breast cancer (3L+) Clinical +
18% 6% 2% 13% 14% 17% 38%