Nature Reviews | Clinical Oncology
Cardiac CT Radiomics MACE Analysis
Phantom studies
An artificial structure that
imitates human tissue
properties is scanned on
multiple machines to
characterize scan output
against a known physical
standard.
comparability of radiomic studies can be achieved only
by extensive disclosure of imaging protocols. We wish
to emphasize this point, and provide examples of how
protocols should be reported in future radiomics studies
(Supplementary informationS1).
Medical imaging
Segmentation. VOIs are segmented manually or (semi-)
automatically
19
. This segmentation determines which vox-
els within an image are analysed, thus, the variability in seg-
mentation can introduce bias in the evaluation of derived
radiomic features
20
. Multiple-segmentation is a method to
limit the extent of this bias. Examples that enable robust
features to be observed
21
include: evaluation by multiple
clinicians, perturb segmentations with noise, combination
of diverse algorithms, or use different stages of the breath-
ing cycle. Key considerations are how the segmentation was
performed, and how sensitive the radiomics analysis is to
different segmentation methods
22
. For example, a semi-
automatic segmentation method can result in different
radiomic features than a manual delineation.
Phantom studies. The determination of inter-scanner and
inter-vendor variability of features is important in radi-
omics
23
. In cases in which radiomic studies rely on data
from multiple scanners, neglecting this variability can
jeopardize the analysis of studies—that is, the proposed
radiomic-based prediction model might not perform ade-
quately on external datasets if new data are acquired on
different scanners. As data from patients scanned on mul-
tiple devices is scarce and subject to uncertainties (such as
organ motion, or different imaging protocols), phantom
studies are a suitable means to gauge these uncertainties
and identify features that rely on the vendor. In essence,
phantom studies provide a risk-mitigation strategy to help
navigate from the current clinical imaging scenario to the
desired optimal imaging scenario.
Imaging at multiple time points. Additional sources
of variability in radiomics features are organ motion or
expansion or shrinkage of the target volume. Radiomics
features that are strongly dependent on these factors can
have limited applicability. To account for these sources of
variability, available test-retest data
24–26
can be exploited
to measure radiomics feature stability. For example, two
datasets of images acquired within a small period of time
from a patientcohort.
Feature extraction
The essence of radiomics is the high-throughput extrac-
tion of quantitative image features to characterize VOIs.
Feature values are dependent upon factors that can
include image pre-processing (for example, filtering, or
intensity discretization) and reconstruction (for exam-
ple, filtered back projection, or iterative reconstruction).
Furthermore, variation exists in feature nomenclature,
mathematical definition, methodology, and software
implementation of the applied feature extraction
algorithms
27–29
. In order to facilitate inter- operability
of radiomic features, differences in nomenclature,
algorithms, software implementations, as well as other
methodological aspects must be elucidated.
Exploratory analysis
Radiomic and non-radiomic features should be com-
bined with the prediction target to create a single
dataset. This approach enables the investigation of
relationships between features. Groups of highly cor-
related radiomics features can be identified via clus-
tering, and these features can be reduced to single
archetypal features per cluster. Radiomic features that
are well-correlated with routine clinical features (such
as tumour stage) do not provide additional information.
Auxiliary feature data collected from multiple segmen-
tations, multiple imaging, and phantom studies, can be
exploited to assess feature robustness. Volatile or robust
features can be identified and subsequently excluded
from model development. For example, a feature that
is robust for the prediction of overall survival for lung
cancer (that is, imaged and segmented in a certain way)
for a given dataset could be volatile for the prediction of
pneumonitis in lung cancer (imaged and segmented in
an alternative way) for a given dataset. Thus, the pro-
cess of feature reduction and/or exclusion should be
described clearly.
Modelling
Radiomic modelling involves three major aspects: fea-
ture selection, modelling methodology, and validation.
Feature selection should be data-driven owing to the
vast in- human range of possible radiomics features; such
analysis should be performed in a robust and transparent
manner. To achieve holistic models, features beyond radi-
omics (such as data from clinical records, data obtained
during treatment or biological and/or genetic) should
also be incorporated. Regarding the choice of modelling
methodology, the identification of optimal machine-
learning methods for radiomic applications is a crucial
step towards stable and clinically relevant CDSS; thus, in
the ideal scenario, multiple machine-learning methods
should be employed
30
and the implementation should be
comprehensively documented. A non-validated model is
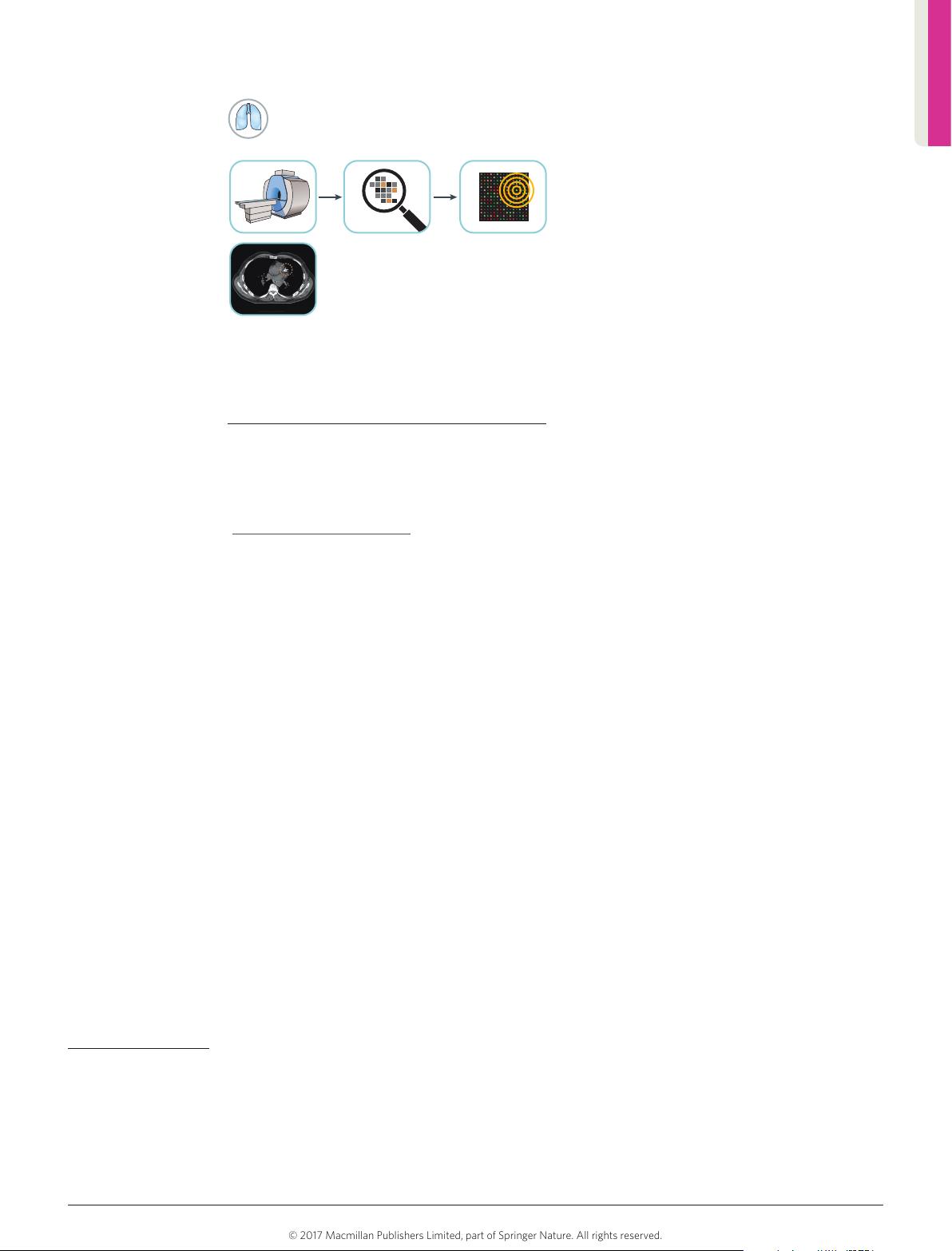
Figure 2
|
Radiomics in cardiology. The current gold standard
for quantification of coronary calcifications visible on CT is the
‘Agatston’ method (based upon intensity and volume).
Radiomic features can improve quantification, differentiation
between calcified and non-calcified plaques, and thus the
prediction of Major Adverse Cardiac Events (MACE).
REVIEWS
NATURE REVIEWS
|
CLINICAL ONCOLOGY VOLUME 14
|
DECEMBER 2017
|
751
©
2
0
1
7
M
a
c
m
i
l
l
a
n
P
u
b
l
i
s
h
e
r
s
L
i
m
i
t
e
d
,
p
a
r
t
o
f
S
p
r
i
n
g
e
r
N
a
t
u
r
e
.
A
l
l
r
i
g
h
t
s
r
e
s
e
r
v
e
d
.
©
2
0
1
7
M
a
c
m
i
l
l
a
n
P
u
b
l
i
s
h
e
r
s
L
i
m
i
t
e
d
,
p
a
r
t
o
f
S
p
r
i
n
g
e
r
N
a
t
u
r
e
.
A
l
l
r
i
g
h
t
s
r
e
s
e
r
v
e
d
.









