yotes. Some editing substrates undergo one or a few
deamination event(s), but a number of RNAs have been
identified that are modified at multiple positions within a
defined region of the primary transcript.
(8)
These cases of so-
called hyperediting, or hypermutation, predominantly involve
viral RNA transcripts such as measles virus and polyoma
virus.
(8,10)
A well-documented non-viral example of hyper-
modification editing is a voltage-dependent potassium
channel RNA (sqKv2) from squid where, in a segment of
360 nucleotides, up to 17 adenosine modifications occur
within a single transcript.
(16)
Functional consequences of RNA editing
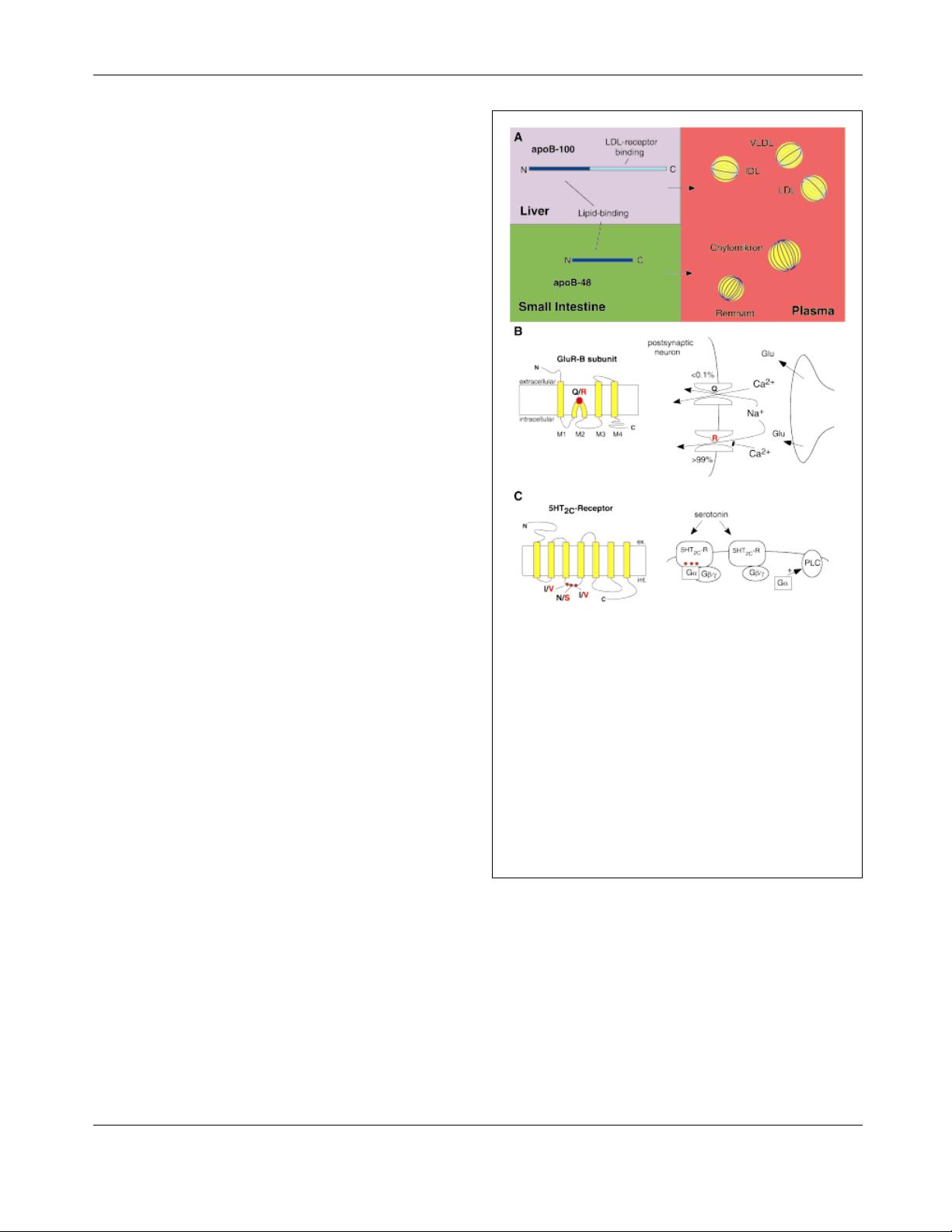
Apolipoprotein B C-to-U editing
Apolipoproteins are essential components of plasma lipo-
proteins that serve as transport vehicles of lipid nutrients in
the circulation.
(17)
The unedited apolipoprotein B transcript
gives rise to full-length apoB (apoB100), which is synthe-
sized in the liver and represents the major protein component
of very low density lipoproteins (VLDL) and their maturation
products intermediate density lipoproteins (IDL) and low
density lipoproteins (LDL). In the small intestine of most
mammals, a truncated apolipoprotein B (apoB48) is pro-
duced as a result of tissue-specific base modification editing
at C6666 and is incorporated into chylomicrons and their
remnants (Fig. 2A). The unedited (apoB100) and edited
(apoB48) variants of apolipoprotein B have quite different
functions in lipid metabolism. Notably, all apoB100-asso-
ciated lipoproteins are highly atherogenic, so that high
plasma levels of VLDL, IDL and LDL particles increase the
susceptability to atherosclerosis. However, apoB48 is a
constituent of lipoproteins of much less atherogenic potential
(chylomicrons and chylomicron remnants). Later we discuss
how understanding the RNA editing mechanism and its
regulation might eventually be used therapeutically to
decrease the risk of atherosclerosis in humans.
A-to-I editing
The functional consequences of A-to-I editing have been
studied most extensively in the glutamate receptor subunit
GluR-B, which was the first A-to-I editing substrate identi-
fied.
(18)
As the major excitatory neurotransmitter in the CNS,
L-glutamate activates three pharmacologically and electro-
physiologically distinct receptor families referred to as NMDA
(N-methyl-D-aspartate), AMPA (alpha-amino-3-hydroxy-5-
methyl-4-isoxazolpropionic acid) and kainate receptors.
Each receptor is assembled from a subset of evolutionary
related protein subunits. Fast excitatory neurotransmission is
mediated by the AMPA receptor channels composed of GluR
subunits A to D. In principal neurons, the putatively
pentameric receptors are characterized by a low Ca
2
-
permeability.
(19)
Through functional studies with recombi-
nantly expressed wild-type and mutant AMPA receptor
channels, this crucial property was demonstrated to be
dependent upon the GluR-B subunit in heteromeric recep-
tors.
(19)
The molecular determinant for the dominant effect of
GluR-B was traced to a single arginine (R) residue located in
the channel-pore lining segment M2 (Fig. 2B).
(18)
The other
subunits, GluR-A, GluR-C and GluR-D carry a glutamine at
the homologous position.
Figure 2. Functional consequences from RNA editing. A:
ApoB100 is produced in the liver and incorporated into VLDL,
IDL and LDL particles. ApoB48 is generated from edited apoB
mRNA in the small intestine and is a major component of
chylomicrons and their remnants. B: Glutamate receptor
subunit GluR-B dominantly regulates the Ca
2
permeability
of AMPA receptors as a result of Q/R site editing. The position
of the Q/R site is indicated by a red dot within the membrane
topology model of GluR-B. In central synapses > 99.9% of
GluR-B subunits carry arginine at this position as the result of
A-to-I editing leading to AMPA channels with low Ca
2
permeability. C: The 5-HT
2C
serotonin receptor topology is
shown with the location of the major A-to-I editing positions
within the second intracellular loop (red dots). G-protein-
coupling efficiency and consequently phospholipase C (PLC)
activation is less efficient with fully edited receptors.
Review articles
792 BioEssays 22.9









